A novel fluorescence-based assay for the rapid detection and quantification of cellular deoxyribonucleoside triphosphates - PubMed (original) (raw)
A novel fluorescence-based assay for the rapid detection and quantification of cellular deoxyribonucleoside triphosphates
Peter M Wilson et al. Nucleic Acids Res. 2011.
Abstract
Current methods for measuring deoxyribonucleoside triphosphates (dNTPs) employ reagent and labor-intensive assays utilizing radioisotopes in DNA polymerase-based assays and/or chromatography-based approaches. We have developed a rapid and sensitive 96-well fluorescence-based assay to quantify cellular dNTPs utilizing a standard real-time PCR thermocycler. This assay relies on the principle that incorporation of a limiting dNTP is required for primer-extension and Taq polymerase-mediated 5-3' exonuclease hydrolysis of a dual-quenched fluorophore-labeled probe resulting in fluorescence. The concentration of limiting dNTP is directly proportional to the fluorescence generated. The assay demonstrated excellent linearity (R(2) > 0.99) and can be modified to detect between ∼0.5 and 100 pmol of dNTP. The limits of detection (LOD) and quantification (LOQ) for all dNTPs were defined as <0.77 and <1.3 pmol, respectively. The intra-assay and inter-assay variation coefficients were determined to be <4.6% and <10%, respectively with an accuracy of 100 ± 15% for all dNTPs. The assay quantified intracellular dNTPs with similar results obtained from a validated LC-MS/MS approach and successfully measured quantitative differences in dNTP pools in human cancer cells treated with inhibitors of thymidylate metabolism. This assay has important application in research that investigates the influence of pathological conditions or pharmacological agents on dNTP biosynthesis and regulation.
Figures
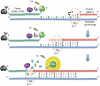
Figure 1.
Simplified schematic illustrating the principle mechanism involved in the fluorescence-based assay for measuring dNTP concentrations. Detection of dTTP using template DT6 is given as the example. The template is depicted in blue, the FAM-dTTP probe in green and primer NDP1 in red. Briefly, as the temperature declines from the 95°C hot-start, the probe anneals to the template first (65–70°C), followed by the primer at 60°C to form the TPP complex at which point Taq polymerase begins extension of the nascent strand. In the presence of a sufficient concentration of limiting dNTP (six dTTP molecules in the example of dTTP-DT6), successful primer extension occurs through the mid-template dNTP detection region and Taq polymerase cleaves the terminal nucleotide labeled with the 6-FAM fluorophore via its 5′-3′ exonuclease activity releasing it from the dual-quenched (ZEN and IBFQ) probe resulting in disruption of FRET and generation of a fluorescence signal in response to excitation-induced photon energy (h_v_). When the dNTP being measured (dTTP) is not present or becomes exhausted, Taq polymerase stalls, extension is inhibited/terminated, fluorescence remains quenched via FRET and the probe remains dark. In any given reaction, the level of fluorescence generated is directly proportional to the concentration of the limiting dNTP. The dAMP molecules enlarged in the template strand represent the nucleotides opposite which the limiting dTTP nucleotides (also enlarged and in bold) will base pair. Only the nucleotide sequence found in the mid-template dNTP detection region is given for simplicity. The complete sequences of all templates (including primer- and probe-binding regions), primer NDP1 and detection probes are given in Table 1. Template, primers and probes were prepared as described in ‘Materials and Methods’ section.
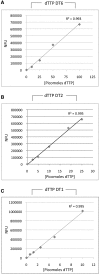
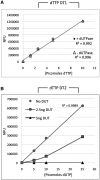
Figure 2.
Validation of dTTP templates with varying detection sensitivities and linear range. Three specific oligonucleotide templates were initially generated and tested for their ability to detect dTTP and tested by calibration curve as described in ‘Materials and Methods’ section. (A) dTTP-DT6 requires the incorporation of six dTTPs for fluorescence generation and yielded a linear range of 0–100 pmol. (B) dTTP-DT2 requires the incorporation of two dTTPs and yielded a linear range of 0–25 pmol. (C) Finally, dTTP-DT1 requires only a single dTTP for incorporation per TPP complex to yield fluorescence and had a linear range of 0.6–10 pmol. Calibration curves for all three templates demonstrated _R_2 of >0.993. In all cases, fluorescence values for blank reactions (limiting dNTP omitted) were subtracted to give NFU.
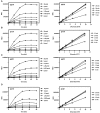
Figure 3.
Validation of dATP, dCTP and dGTP detection templates. Calibration curves were generated for dNTPs using dNTP-specific templates (DT1 and DT2) and probes (Table 1) and were performed as described in ‘Materials and Methods’ section. In all cases, fluorescence values for blank reactions (limiting dNTP omitted) were subtracted to give NFU. All curves demonstrated _R_2 > 0.99. (A) dATP, (B) dCTP, (C) dGTP. Left, DT1; Right, DT2.
Figure 4.
Time-course and calibration curve analysis of the polymerase reaction. Time course showing fluorescence generated by the dNTP-dependent Taq DNA polymerase-mediated hydrolysis of a dual-quenched fluorescent-labeled probe. Left. Known pmole quantities of dNTP were detected using DT1 and fluorescence was analyzed at 5-min intervals on board an Applied Biosystems 7500 Real-Time PCR System. Right. Calibration curves were generated and plotted from the NFU obtained at the specified time intervals and analyzed by linear regression. All calibration curves demonstrated _R_2 of >0.99. (A) dGTP. (B) dTTP. (C) dATP. (D) dCTP. Additional details of the assay are described in the ‘Materials and Methods’ section.
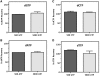
Figure 5.
Effect of a 100- and 1000-fold molar rNTP excess on the recovery of dNTPs in the fluorescence-based assay with Taq polymerase. (A) The recovery of 5 pmol of dGTP was determined in the presence of both a 100- and 1000-fold molar excess of GTP at assay completion (20 min). The same analysis was applied to the recovery of (B) dATP, (C) dCTP and (D) dTTP in the presence of their corresponding rNTP. Bars represent the mean + SD of three individual analyses. The assay was performed as described in ‘Materials and Methods’ section. In all cases, fluorescence values for blank reactions (limiting dNTP omitted) were subtracted to give NFUs.
Figure 6.
Detection of dUTP. The ability of the assay to detect dUTP and distinguish dTTP from dUTP in the presence and absence of the dUTP-hydrolyzing enzyme dUTPase was analyzed. (A) The effects of including recombinant human dUTPase (DUT) and a 5 min pre-incubation at 37°C were first analyzed. Inclusion of 5 ng of dUTPase had no significant impact on the assay performance and detection of dTTP (_R_2 > 0.99). (B) dTTP was replaced with dUTP and the reaction performed in the absence of dUTPase and in the presence of 2.5 and 5 ng of recombinant human dUTPase. In the absence of dUTPase, dUTP detection was robust and yielded an excellent calibration curve (_R_2 > 0.99). Five nanograms of dUTPase was sufficient to eliminate dUTP as the source of fluorescence in the assay, whereas 2.5 ng resulted in partial hydrolysis and intermediate fluorescence. The assay was performed as described in ‘Materials and Methods’ section. In all cases, fluorescence values for blank reactions (limiting dNTP omitted) were subtracted to give NFUs.
Similar articles
- Quantification of Cellular Deoxyribonucleoside Triphosphates by Rolling Circle Amplification and Förster Resonance Energy Transfer.
Qiu X, Guittet O, Mingoes C, El Banna N, Huang ME, Lepoivre M, Hildebrandt N. Qiu X, et al. Anal Chem. 2019 Nov 19;91(22):14561-14568. doi: 10.1021/acs.analchem.9b03624. Epub 2019 Nov 1. Anal Chem. 2019. PMID: 31638767 - Quantitation of cellular deoxynucleoside triphosphates.
Ferraro P, Franzolin E, Pontarin G, Reichard P, Bianchi V. Ferraro P, et al. Nucleic Acids Res. 2010 Apr;38(6):e85. doi: 10.1093/nar/gkp1141. Epub 2009 Dec 11. Nucleic Acids Res. 2010. PMID: 20008099 Free PMC article. - A user-friendly, high-throughput tool for the precise fluorescent quantification of deoxyribonucleoside triphosphates from biological samples.
Szabó JE, Surányi ÉV, Mébold BS, Trombitás T, Cserepes M, Tóth J. Szabó JE, et al. Nucleic Acids Res. 2020 May 7;48(8):e45. doi: 10.1093/nar/gkaa116. Nucleic Acids Res. 2020. PMID: 32103262 Free PMC article. - DNA precursor metabolism and genomic stability.
Mathews CK. Mathews CK. FASEB J. 2006 Jul;20(9):1300-14. doi: 10.1096/fj.06-5730rev. FASEB J. 2006. PMID: 16816105 Review.
Cited by
- NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to Mediate the Anticancer Efficacy of 6-Thioguanine.
Valerie NC, Hagenkort A, Page BD, Masuyer G, Rehling D, Carter M, Bevc L, Herr P, Homan E, Sheppard NG, Stenmark P, Jemth AS, Helleday T. Valerie NC, et al. Cancer Res. 2016 Sep 15;76(18):5501-11. doi: 10.1158/0008-5472.CAN-16-0584. Epub 2016 Aug 16. Cancer Res. 2016. PMID: 27530327 Free PMC article. - dTMP imbalance through thymidylate 5'-phosphohydrolase activity induces apoptosis in triple-negative breast cancers.
Kim DH, Kim JS, Mok CS, Chang EH, Choi J, Lim J, Kim CH, Park AR, Bae YJ, Koo BS, Lee HC. Kim DH, et al. Sci Rep. 2022 Nov 21;12(1):20027. doi: 10.1038/s41598-022-24706-4. Sci Rep. 2022. PMID: 36414668 Free PMC article. - Equine lentivirus counteracts SAMHD1 restriction by Rev-mediated degradation of SAMHD1 via the BECN1-dependent lysosomal pathway.
Ren H, Yin X, Su C, Guo M, Wang XF, Na L, Lin Y, Wang X. Ren H, et al. Autophagy. 2021 Oct;17(10):2800-2817. doi: 10.1080/15548627.2020.1846301. Epub 2020 Nov 24. Autophagy. 2021. PMID: 33172327 Free PMC article. - Fast and sensitive HPLC-MS/MS method for direct quantification of intracellular deoxyribonucleoside triphosphates from tissue and cells.
Olafsson S, Whittington D, Murray J, Regnier M, Moussavi-Harami F. Olafsson S, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Nov 15;1068-1069:90-97. doi: 10.1016/j.jchromb.2017.10.008. Epub 2017 Oct 5. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 29032043 Free PMC article. - Quantitative Analysis of Nucleoside Triphosphate Pools in Mouse Muscle Using Hydrophilic Interaction Liquid Chromatography Coupled with Tandem Mass Spectrometry Detection.
Sharma S, Kong Z, Jia S, Tran P, Nilsson AK, Chabes A. Sharma S, et al. Methods Mol Biol. 2023;2615:267-280. doi: 10.1007/978-1-0716-2922-2_19. Methods Mol Biol. 2023. PMID: 36807798
References
- Kunz BA, Kohalmi SE, Kunkel TA, Mathews CK, McIntosh EM, Reidy JA. International Commission for protection against environmental mutagens and carcinogens. Deoxyribonucleoside triphosphate levels: a critical factor in the maintenance of genetic stability. Mutat. Res. 1994;318:1–64. - PubMed
- Smid K, Van Moorsel CJ, Noordhuis P, Voorn DA, Peters GJ. Interference of gemcitabine triphosphate with the measurements of deoxynucleotides using an optimized DNA polymerase elongation assay. Int. J. Oncol. 2001;19:157–162. - PubMed
- Horowitz RW, Zhang H, Schwartz EL, Ladner RD, Wadler S. Measurement of deoxyuridine triphosphate and thymidine triphosphate in the extracts of thymidylate synthase-inhibited cells using a modified DNA polymerase assay. Biochem. Pharmacol. 1997;54:635–638. - PubMed
- Sherman PA, Fyfe JA. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal. Biochem. 1989;180:222–226. - PubMed
- Skoog L. An enzymatic method for the determination of dCTP and dGTP in picomole amounts. Eur. J. Biochem. 1970;17:202–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources