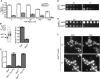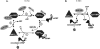The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1 - PubMed (original) (raw)
The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1
Kristen N Noble et al. Genes Dev. 2011.
Abstract
Essential messenger RNA (mRNA) export factors execute critical steps to mediate directional transport through nuclear pore complexes (NPCs). At cytoplasmic NPC filaments, the ATPase activity of DEAD-box protein Dbp5 is activated by inositol hexakisphosphate (IP(6))-bound Gle1 to mediate remodeling of mRNA-protein (mRNP) complexes. Whether a single Dbp5 executes multiple remodeling events and how Dbp5 is recycled are unknown. Evidence suggests that Dbp5 binding to Nup159 is required for controlling interactions with Gle1 and the mRNP. Using in vitro reconstitution assays, we found here that Nup159 is specifically required for ADP release from Dbp5. Moreover, Gle1-IP(6) stimulates ATP binding, thus priming Dbp5 for RNA loading. In vivo, a dbp5-R256D/R259D mutant with reduced ADP binding bypasses the need for Nup159 interaction. However, NPC spatial control is important, as a dbp5-R256D/R259D nup42Δ double mutant is temperature-sensitive for mRNA export. Further analysis reveals that remodeling requires a conformational shift to the Dbp5-ADP form. ADP release factors for DEAD-box proteins have not been reported previously and reflect a new paradigm for regulation. We propose a model wherein Nup159 and Gle1-IP(6) regulate Dbp5 cycles by controlling its nucleotide-bound state, allowing multiple cycles of mRNP remodeling by a single Dbp5 at the NPC.
Figures
Figure 1.
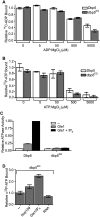
Nup159 promotes Dbp5 release of ADP. (A) Equilibrium binding assays show an increase in initial rate of Dbp5–14C-ADP binding: Dbp5 (1 μM) was incubated with increasing concentrations of 14C-ADP (0.5–150 μM) over the course of 24 h. Samples were removed at several time points and used in filter-binding assays. Background integrated density signals of 14C-ADP at each concentration were subtracted from the Dbp5-bound ADP signal. As listed in the table, initial velocities of binding (fraction 14C-ADP bound per minute) were calculated during the linear phase of the binding curve at each Dbp5:14C-ADP ratio tested. (B) Dbp5 does not release bound ADP after equilibration: After 24-h equilibration of 1 μM Dbp5 and 5 μM 14C-ADP:MgCl2, excess unlabeled ATP:MgCl2 or ADP:MgCl2 (10 mM of each) was added, and filter-binding assays were conducted after an additional 24 h incubation. (C) Nup159-NTD does not promote release of the nonhydrolyzable ATP analog ADP-BeFx: After 26-h equilibration of 1 μM Dbp5 with 5 μM 14C-ADP-BeFx, either 1 μM Nup159NTD, 500 nM Gle1 with 200 nM IP6, or 2 μM poly(A)25 RNA was added, and filter-binding assays were performed at 24 h after addition. (D) Nup159-NTD specifically promotes Dbp5 release of bound ADP: After 24-h equilibration of 1 μM Dbp5 with 5 μM 14C-ADP:MgCl2, either 1 μM Nup159-NTD, 500 nM Gle1 with 200 nM IP6, or 2 μM RNA was added, and filter-binding assays were performed at 10 min and 24 h after addition. Two altered nup159 proteins (nup159DD and nup159VI) that do not bind Dbp5 in vitro were used as negative controls. (E) Increasing concentration of Nup159 promotes faster Dbp5 release of bound 14C-ADP. Dbp5 (1 μM) was incubated with 5 μM 14C-ADP for 24 h. Increasing concentrations of Nup159 were added and filter-binding assays were performed over the course of 2 h. All data points and bars represent at least three independent experiments with standard error bars.
Figure 2.
Gle1-IP6 enhances Dbp5 binding to ATP. (A,B) Adenine nucleotide binding of Dbp5 versus dbp5EQ: Filter-binding assays were conducted to assay for relative binding to ATP and ADP. Purified recombinant Dbp5 or dbp5EQ (1 μM) was incubated with 1 μM 32P-ATP:MgCl2 or 14C-ADP:MgCl2 and increasing concentrations of unlabeled nucleotide ranging from 0 nM to 5000 nM. Bars represent the averages of three independent experiments with standard error shown. (C) dbp5EQ is inhibited for ATPase activity: Colorimetric ATPase-coupled enzyme assays were performed with purified recombinant 500 nM Dbp5 or dbp5EQ, 1 mM ATP:MgCl2, and 1 μM poly(A)25 RNA. Gle1 (250 nM) was added with or without 100 nM IP6. (D) Gle1-IP6 promotes ATP loading of dbp5E240Q: dbp5E240Q (2 μM) was incubated with 10 nM α32P-ATP in the presence of 2 μM Nup159-NTD, 1 μM Gle1 with 400 nM IP6, or 4 μM poly(A)25 RNA for 4 h. Filter-binding assays were performed. In all panels, bars represent the averages of three independent experiments with standard error bars.
Figure 3.
dbp5RR mutant does not bind Nup159 and displays no in vivo growth defects. (A) Amino acid residue alignment for a portion of S. cerevisiae Dbp5 and Homo sapiens hDBP5. Black stars designate conserved arginine residues that were changed to aspartic acid residues (R256D and R259D) in Dbp5 to yield dbp5RR. The open white star represents a conserved glutamate residue that was changed to glutamine (E240Q) to yield dbp5EQ. Conserved DEAD-box family sequence motifs are boxed and identified below alignment. (B) Cytoplasmic mislocalization of GFP-dbp5RR by live-cell microscopy: S. cerevisiae strains with DBP5 chromosomally deleted, expressing either GFP-DBP5 or GFP-dbp5RR on plasmids, were used in live-cell fluorescent microscopy experiments to visualize subcellular localization of each protein. (Left) DIC image. (Right) GFP. Western blot analysis of yeast whole-cell extracts was conducted to test for relatively equal levels of expression of GFP-Dbp5 and GFP-dbp5RR. Nup116 protein level was used as a loading control. (*) Degradation products of Nup116. (C) Nup159-NTD interaction in vitro with dbp5RR is not detected: Soluble binding assays were conducted with 200 pmol of bacterially expressed recombinant His6-Nup159-NTD and immobilized on Ni-NTA agarose resin and either recombinant Dbp5, dbp5RR, or dbp5EQ. Input and bound fractions were analyzed by SDS-PAGE and Coomassie staining. (*) Nup159-NTD degradation product. (D) Swapping the salt bridge interaction allows nup159DD and dbp5RR interaction: Soluble binding assays were conducted as in C with His6-Nup159-NTD, His6-nup159VI-NTD, His6-nup159DD-NTD, Dbp5, or dbp5RR. (E) dbp5RR strain does not have growth defects: S. cerevisiae strains with DBP5 chromosomally deleted, expressing either wild-type DBP5 or dbp5RR, were used to perform serial dilution growth assays at 23°C, 30°C, and 37°C.
Figure 4.
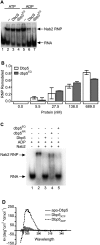
dbp5RR does not require Nup159 for ADP release. (A) dbp5RR has reduced binding affinity to ADP as compared with wild-type Dbp5: Dbp5 (1 μM) or dbp5RR (1 μM) was incubated with 5 μM 14C-ADP and increasing concentrations of unlabeled ADP:MgCl2. Filter-binding assays were performed to measure the relative fraction of ADP bound to each protein. Bars represent the averages of three independent experiments with standard error bars. (B) dbp5RR does not require Nup159 for ADP release: α32P-ATP was incubated with either Dbp5 or dbp5RR and 400 μM poly(A)25 RNA for 24 h to allow complete hydrolysis to α32P-ADP to occur. After 24 h, samples for filter-binding assays were removed and the remaining reaction was stopped with proteinase K digestion. (Left) The digested samples were spotted onto thin-layer chromatography plates to monitor ATP hydrolysis. (Right) The portion of the samples removed for filter-binding assays were used to monitor the relative amount of ADP still bound to either Dbp5 or dbp5RR. Bars represent the averages of three independent experiments with standard error shown. (C) dbp5RR is active for ATP hydrolysis and Gle1-IP6 stimulation: Colorimetric ATPase-coupled enzyme assays were performed as described in Figure 1B with 500 nM Dbp5 or 500 nM dbp5RR. Gle1 (250 nM) with 100 nM IP6 was added to measure relative levels of stimulation. The ATPase activity of each sample was normalized to the maximally stimulated Dbp5 with Gle1-IP6 sample. Bars represent the averages of three independent experiments with standard error shown. (D) The dbp5RR mutant complements the rat7ΔN temperature sensitivity. Yeast strains were grown at the permissive temperature and serially diluted on plates for growth at 23°C and 37°C. (E) The dbp5RR nup42Δ double mutant has a synthetic fitness growth defect. Yeast strains were grown at the permissive temperature and serially diluted on plate for growth at 23°C and 37°C. (F) Nuclear poly(A) RNA accumulates in arrested dbp5RR nup42Δ cells. In situ hybridization with an oligo(dT) probe for poly(A) RNA localization was conducted on dbp5RR nup42Δ cells grown at 23°C and shifted to growth for 1 h at 37°C. DAPI staining marks the nucleus (left) and fluorescent signal for the poly(A) RNA (right).
Figure 5.
Dbp5 remodeling mechanism is mediated by a conformational change. (A) In vitro remodeling assay demonstrates that dbp5EQ remodels similarly to wild-type Dbp5: In vitro constituted Nab2-RNPs were assembled as described in Tran et al. (2007) with 5′-end labeled 32P-poly(A)25. Nab2 (250 nM) was incubated with 10 nM RNA and 500 nM either wild-type Dbp5 or dbp5EQ in the presence of 1 mM ATP:MgCl2 or 1 mM ADP:MgCl2. Electrophoretic mobility shift assays (EMSAs) were performed and products were visualized by autoradiography. (B) Quantification of Nab2-RNPs remodeling: Increasing concentrations of Dbp5 and dbp5EQ were added to in vitro constituted Nab2-RNPs in the presence of ADP:MgCl2 and filter-binding assays were performed. Densitometry was used to quantify the fraction of Nab2-RNPs that were remodeled at each concentration of protein. Bars represent the averages of three independent experiments with standard error bars. (C) dbp5RR remodels in vitro constituted Nab2-RNPs less efficiently than wild-type Dbp5: EMSAs were performed as described above using 750 nM wild-type Dbp5, dbp5E240Q, or dbp5RR in the presence of 1 mM ADP:MgCl2. The gel was exposed to autoradiography to detect the relative Nab2-RNP and free RNA. (D) Circular dichroism studies demonstrate nucleotide-dependent conformational changes: Near-UV spectral scans of Dbp5 with 1.5 mM ATP:MgCl2, ADP:MgCl2, or MgCl2 alone were collected from 250-nm to 350-nm wavelengths. Spectra of buffer with 1.5 mM ATP:MgCl2, ADP:MgCl2, or MgCl2 were subtracted from the Dbp5 spectra to remove interfering signals from the adenosine moiety. Θ represents molar elipticity, which is calculated as (100 × signal)/(pathlength × amino acid residues × mM concentration).
Figure 6.
Proposed mechanism of Dbp5 nucleotide cycle. The left panel illustrates in vivo steps, and nucleotide binding/hydrolysis are controlled by multiple binding partners. (Step 1) Bound to ATP and an mRNP, Dbp5 is primed for remodeling. Hydrolysis is stimulated by Gle1-IP6, resulting in release of Pi and the remodeled mRNP, and generation of a conformationally changed ADP-bound Dbp5. (Step 2) Bound to ADP, Nup159 promotes ADP release, which confers another conformational change and places Dbp5 in an APO state. (Step 3) Gle1-IP6 promotes the loading of ATP to allow another round of nucleotide cycling and remodeling. (Step 4) Bound to ATP, Dbp5 has the highest affinity for RNA. RNA binding and ATP hydrolysis release Gle1-IP6. The right panel proposes a model for in vitro mimicking via ADP binding to the APO form of Dbp5, allowing mRNP remodeling in the absence of Gle1-IP6. Once bound to ADP in vitro, Nup159 promotes nucleotide release.
Comment in
- Regulation of the Dbp5 ATPase cycle in mRNP remodeling at the nuclear pore: a lively new paradigm for DEAD-box proteins.
Ledoux S, Guthrie C. Ledoux S, et al. Genes Dev. 2011 Jun 1;25(11):1109-14. doi: 10.1101/gad.2062611. Genes Dev. 2011. PMID: 21632821 Free PMC article.
Similar articles
- The Dbp5 cycle at the nuclear pore complex during mRNA export I: dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1.
Hodge CA, Tran EJ, Noble KN, Alcazar-Roman AR, Ben-Yishay R, Scarcelli JJ, Folkmann AW, Shav-Tal Y, Wente SR, Cole CN. Hodge CA, et al. Genes Dev. 2011 May 15;25(10):1052-64. doi: 10.1101/gad.2041611. Genes Dev. 2011. PMID: 21576265 Free PMC article. - Nucleoporin FG domains facilitate mRNP remodeling at the cytoplasmic face of the nuclear pore complex.
Adams RL, Terry LJ, Wente SR. Adams RL, et al. Genetics. 2014 Aug;197(4):1213-24. doi: 10.1534/genetics.114.164012. Epub 2014 Jun 14. Genetics. 2014. PMID: 24931410 Free PMC article. - Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export.
Folkmann AW, Noble KN, Cole CN, Wente SR. Folkmann AW, et al. Nucleus. 2011 Nov-Dec;2(6):540-8. doi: 10.4161/nucl.2.6.17881. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064466 Free PMC article. Review. - Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export.
Alcázar-Román AR, Tran EJ, Guo S, Wente SR. Alcázar-Román AR, et al. Nat Cell Biol. 2006 Jul;8(7):711-6. doi: 10.1038/ncb1427. Epub 2006 Jun 18. Nat Cell Biol. 2006. PMID: 16783363 - Dbp5 - from nuclear export to translation.
Tieg B, Krebber H. Tieg B, et al. Biochim Biophys Acta. 2013 Aug;1829(8):791-8. doi: 10.1016/j.bbagrm.2012.10.010. Epub 2012 Nov 2. Biochim Biophys Acta. 2013. PMID: 23128325 Review.
Cited by
- Sending the message: specialized RNA export mechanisms in trypanosomes.
Obado SO, Rout MP, Field MC. Obado SO, et al. Trends Parasitol. 2022 Oct;38(10):854-867. doi: 10.1016/j.pt.2022.07.008. Epub 2022 Aug 24. Trends Parasitol. 2022. PMID: 36028415 Free PMC article. Review. - Cofactor-dependent specificity of a DEAD-box protein.
Young CL, Khoshnevis S, Karbstein K. Young CL, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):E2668-76. doi: 10.1073/pnas.1302577110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630256 Free PMC article. - Nucleocytoplasmic shuttling of Gle1 impacts DDX1 at transcription termination sites.
Sharma M, Wente SR. Sharma M, et al. Mol Biol Cell. 2020 Oct 1;31(21):2398-2408. doi: 10.1091/mbc.E20-03-0215. Epub 2020 Aug 5. Mol Biol Cell. 2020. PMID: 32755435 Free PMC article. - Insights into mRNP biogenesis provided by new genetic interactions among export and transcription factors.
Estruch F, Hodge C, Gómez-Navarro N, Peiró-Chova L, Heath CV, Cole CN. Estruch F, et al. BMC Genet. 2012 Sep 10;13:80. doi: 10.1186/1471-2156-13-80. BMC Genet. 2012. PMID: 22963203 Free PMC article. - RNA Export through the NPC in Eukaryotes.
Okamura M, Inose H, Masuda S. Okamura M, et al. Genes (Basel). 2015 Mar 20;6(1):124-49. doi: 10.3390/genes6010124. Genes (Basel). 2015. PMID: 25802992 Free PMC article. Review.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. 2007. The molecular architecture of the nuclear pore complex. Nature 450: 695–701 - PubMed
- Alcazar-Roman AR, Tran EJ, Guo S, Wente SR 2006. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol 8: 711–716 - PubMed
- Andreasson C, Fiaux J, Rampelt H, Mayer MP, Bukau B 2008. Hsp110 is a nucleotide-activated exchange factor for Hsp70. J Biol Chem 283: 8877–8884 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA119925/CA/NCI NIH HHS/United States
- R01 GM33998/GM/NIGMS NIH HHS/United States
- F31 HD061181/HD/NICHD NIH HHS/United States
- 1F31 HD061181/HD/NICHD NIH HHS/United States
- F32 GM075459/GM/NIGMS NIH HHS/United States
- R37 GM051219/GM/NIGMS NIH HHS/United States
- R01 GM033998/GM/NIGMS NIH HHS/United States
- 1F32 GM075459/GM/NIGMS NIH HHS/United States
- R37 GM51219/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases