Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca²⁺ wave-dependent activation of SK and TRPC channels - PubMed (original) (raw)
Comparative Study
Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca²⁺ wave-dependent activation of SK and TRPC channels
Lynda El-Hassar et al. J Physiol. 2011.
Abstract
Group I metabotropic glutamate receptors (mGluRs) play an essential role in cognitive function. Their activation results in a wide array of cellular and molecular responses that are mediated by multiple signalling cascades. In this study, we focused on Group I mGluR activation of IP3R-mediated intracellular Ca2+ waves and their role in activating Ca2+-dependent ion channels in CA1 pyramidal neurons. Using whole-cell patch-clamp recordings and high-speed Ca2+ fluorescence imaging in acute hippocampal brain slices, we show that synaptic and pharmacological stimulation of mGluRs triggers intracellular Ca2+ waves and a biphasic electrical response composed of a transient Ca2+-dependent SK channel-mediated hyperpolarization and a TRPC-mediated sustained depolarization. The generation and magnitude of the SK channel-mediated hyperpolarization depended solely on the rise in intracellular Ca2+ concentration ([Ca2+]i), whereas the TRPC channel-mediated depolarization required both a small rise in [Ca2+]i and mGluR activation. Furthermore, the TRPC-mediated current was suppressed by forskolin-induced rises in cAMP. We also show that SK- and TRPC-mediated currents robustly modulate pyramidal neuron excitability by decreasing and increasing their firing frequency, respectively. These findings provide additional evidence that mGluR-mediated synaptic transmission makes an important contribution to regulating the output of hippocampal neurons through intracellular Ca2+ wave activation of SK and TRPC channels. cAMP provides an additional level of regulation by modulating TRPC-mediated sustained depolarization that we propose to be important for stabilizing periods of sustained firing.
Figures
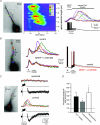
Figure 1. Synaptically evoked intracellular Ca2+ waves and an associated biphasic membrane potential change in CA1 pyramidal neurons
A, left panel, fluorescence image of a fura-2FF-filled neuron. Middle and right panels, two representations of the same imaging data elicited by synaptic stimulation (50 pulses at 100 Hz; see electrical response in right panel). The middle panel is a pseudo-linescan showing that intracellular Ca2+ waves propagate through hot and cold spots of release. The right panel shows coloured waveforms corresponding to Ca2+ rises occurring at the colour-coded boxes (regions of interest, ROIs) over the cell in the left panel. Note the difference in magnitude and timing of the VGCC-mediated Ca2+ rise occurring during the synaptically elicited action potentials and the delayed intracellular Ca2+ wave. B, Group I mGluR blockers selectively blocked intracellular Ca2+ waves, but not VGCC-mediated rises in [Ca2+]i. Rises in [Ca2+]i were first initiated by activation of VGCCs with current injection-evoked spikes (2 ms, 2 nA, 10 spikes at 100 Hz) followed by synaptic stimulation (30 pulses at 100 Hz). Middle panel, mGluR antagonists, MPEP (10 μ
m
) and LY367385 (100 μ
m
), blocked synaptically elicited internal Ca2+ release. Right panel, the evoked electrical waveforms recorded during the Ca2+ responses shown in the middle panel. Note the suppression of the membrane depolarization following bath application of the mGluR antagonists. C, synaptically elicited intracellular Ca2+ waves correlate with a biphasic membrane potential change. Synaptic stimulation, subthreshold for eliciting a rise in [Ca2+]i (100 pulses at 100 Hz), failed to elicit a biphasic membrane potential change. 30 s after ‘priming’ the cell with a train of current injection-evoked spikes (2 ms, 2 nA current injection; 100 spikes at 100 Hz) and consequent VGCC-mediated Ca2+ influx (not shown), the previously subthreshold synaptic stimulation elicited internal Ca2+ release and a hyperpolarization and depolarization. Note that the fast EPSPs evoked by electrical stimulation were not affected by priming. In this example, mAChRs and GABABRs were blocked (1 μ
m
atropine and 1 μ
m
CGP55845, respectively). D, summary data showing priming-induced facilitation of synaptically elicited internal Ca2+ release and associated membrane potential changes (normalized to pre-priming response averages; n = 7; *P < 0.01, **P < 0.001; ANOVA).
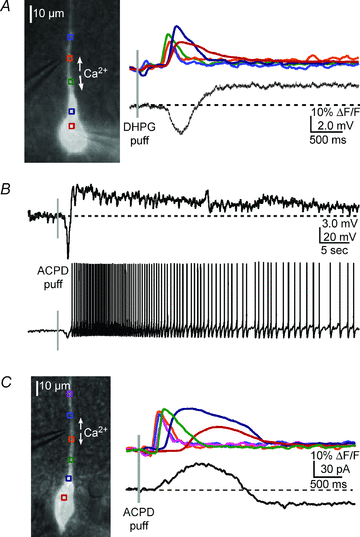
Figure 2. mGluR agonists elicit a Ca2+ wave-dependent hyperpolarization and depolarization
A, left, overlay of a DIC image and fluorescence neuron image showing the position of the pressure application pipette near the primary apical dendrite of the recorded cell. Right, a DHPG puff (400 μ
m
, 50 ms) onto the primary apical dendrite triggered a bidirectionally propagating Ca2+ wave and associated hyperpolarization and depolarization of a CA1 pyramidal neuron. B, upper panel, in a different neuron, an ACPD puff (400 μ
m
, 50 ms) elicited a Ca2+ wave (not shown) and a transient hyperpolarization and a sustained depolarization. Lower panel, when the neuron was held at a membrane potential slightly subthreshold for spiking (∼−53 mV), ACPD elicited a transient hyperpolarization and a sustained train of action potentials. C, in voltage clamp, an ACPD puff elicited an outward current and inward current (see Results for summary data).
Figure 3. The hyperpolarization and depolarization are due to SK channels and CAN channels, respectively
A, left, the reversal potential (_E_rev) of the hyperpolarizing potential was determined to be ∼−85 mV by applying DHPG puffs (400 μ
m
, 50 ms) at different holding potentials in current clamp and measuring membrane potential changes. Intracellular Ca2+ waves were similar at all holding potentials (waves are colour-coded by holding potential). Right panel, summary graph showing the reversal potential for all cells tested (n = 5; each cell is represented by a different colour). B, consistent with a mechanism involving SK channels, apamin (100 n
m
) blocked the hyperpolarization. This treatment unveiled the isolated depolarizing potential and revealed its delayed onset. C, left and middle panels, the mGluR-mediated, Ca2+-dependent depolarizing current was isolated in voltage clamp and its _E_rev was determined to be ∼12 mV. DHPG puffs (400 μ
m
, 50 ms) were delivered to the primary apical dendrite in the presence of voltage-gated K+ channel and Na+ channel blockers, and GABABR blockers (see Results). Right, summary I–V graph for all cells tested (n = 5; each cell is represented by a different colour) shows data consistent with activation of CAN channels.
Figure 4. The sustained depolarization requires both group I mGluR receptor activation and a rise in [Ca2+]i
A, fluorescence image of a neuron filled with fluo-4 (100 μ
m
) and NPE-caged IP3 (97 μ
m
). Coloured boxes indicate the regions of interest ROIs in apical dendrites corresponding to the optical traces showing internal Ca2+ release on the right. UV flashes directed at the proximal primary apical dendrite (20 μm diameter, 400 ms duration; represented by yellow circle) elicited internal Ca2+ release and a hyperpolarizing potential, but not a depolarization. B, a caffeine puff (50 m
m
, 50 ms) onto the proximal apical dendrite elicited intracellular Ca2+ waves and a transient hyperpolarization, but no depolarization. C, the sustained depolarization depended on mGluR activation and a rise in [Ca2+]. Depleting Ca2+ stores with CPA (50 μ
m
) prevented mGluR-mediated internal Ca2+ release and membrane potential changes. In the absence of internal Ca2+ release, pairing mGluR activation with VGCC-mediated Ca2+ influx during spikes evoked by current injection (2 ms, 2 nA current injections; 10–50 spikes at 100 Hz) elicited a similar sustained depolarization. Under these conditions, spiking elicited a VGCC-mediated hyperpolarization that was not affected by agonist application (data not shown). D, summary data showing rescue of membrane potential changes when internal stores are depleted (n = 5, **P < 0.001, ANOVA).
Figure 5. Pharmacological characterization of the sustained depolarization—non-specific blockers of _I_CAN/_I_TRPC suppressed the sustained depolarization
A, internal Ca2+ stores were first depleted with CPA (50 μ
m
). VGCC-mediated rises in [Ca2+]i were elicited with current-injected spikes and paired with puffs of DHPG. Addition of both flufenamate (FFA; 100 μ
m
) and SKF96365 (30 μ
m
) suppressed both the depolarization and the hyperpolarization (n = 5; P < 0.01, t test). B, addition of FFA (100 μ
m
) alone blocked the depolarization (n = 3). C, addition of SKF96365 (30–100 μ
m
) alone had no effect on either the depolarization or the hyperpolarization under these conditions (n = 5).
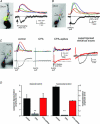
Figure 6. TRPC1, TRPC4 and TRPC5 antibodies block the mGluR-mediated and intracellular Ca2+ wave-dependent depolarization
Antibodies to TRPC were loaded into patch recording pipettes (1:100 dilution). In some cases antibodies were heat inactivated. Responses recorded ∼5 min after breaking into the cell were compared to responses recorded ∼20 min after breaking in. A, an example of data collected from a CA1 pyramidal neuron loaded with anti-TRPC1 and an example of a neuron loaded with heat-inactivated anti-TRPC1. Anti-TRPC1 selectively blocked the sustained depolarization. B, examples of neurons loaded with anti-TRPC3, anti-TRPC4 or anti-TRPC5. TRPC3 did not affect the depolarization (n = 3, P > 0.1, t test); TRPC4 (n = 5), like TRPC1 (n = 5) and TRPC5 (n = 5; data not shown), suppressed the mGluR/IP3R evoked-depolarization (P < 0.01 for each antibody, t test). C, summary data for anti-TRPCs, and the controls, IgG (n = 7) or inactivated anti-TRPC1 (n = 5) or TRPC5 (n = 5).
Figure 7. Rises in intracellular cAMP differentially suppressed the mGluR-mediated depolarization and hyperpolarization
A, DHPG application paired with current injection-evoked action potentials elicited robust rises in [Ca2+]i and associated membrane potential changes. Bath application of forskolin (5–10 μ
m
) totally blocked the TRPC channel-mediated depolarization. Forskolin also partially suppressed the SK channel-mediated AHP, but not the fast Ca2+-dependent AHP. Forskolin also suppressed internal Ca2+ release in this cell. B, summary data showing suppression of the depolarization (n = 9; *P < 0.01, t test).
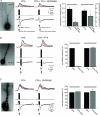
Figure 8. TRPC1, 4 and 5 antibodies suppress mGluR-mediated increases in spike frequency
A, mGluR activation of intracellular Ca2+ waves modulates the firing pattern of CA1 pyramidal neurons. Puffing DHPG (50 ms) onto the apical dendrite of a spiking pyramidal neuron held at ∼−45 mV in current clamp suppressed and then increased the firing frequency of this representative pyramidal neuron. B, an example of a neuron loaded with IgG. mGluR regulation of firing frequency was not affected by IgG (or inactivated anti-TRPCs; not shown). C, an example of a neuron loaded with anti-TRPC4. Addition of anti-TRPC1, 4 or 5 (1:100 dilution) to the patch pipette suppressed the increase in firing frequency 20 min after breaking into the cell. D, summary data showing that anti-TRPC1, 4 and 5 suppress mGluR-mediated increases in firing frequency (cumulatively P < 0.01, _t_ test). TRPC3 (_n_ = 3) did not alter mGluR-mediated regulation of firing (_P_ > 0.1, t test). Inactivated anti-TRPCs (n = 4) and IgG (n = 7) did not have a significant effect on mGluR-mediated increases in firing rate (P > 0.1, t test). Caffeine puffs (50 m
m
; n = 5), which activate an RyR-mediated _I_SK but not an _I_TRPC, did not elicit an increase in firing frequency (P > 0.1, t test).
Similar articles
- Disrupted in schizophrenia 1 modulates medial prefrontal cortex pyramidal neuron activity through cAMP regulation of transient receptor potential C and small-conductance K+ channels.
El-Hassar L, Simen AA, Duque A, Patel KD, Kaczmarek LK, Arnsten AF, Yeckel MF. El-Hassar L, et al. Biol Psychiatry. 2014 Sep 15;76(6):476-85. doi: 10.1016/j.biopsych.2013.12.019. Epub 2014 Jan 20. Biol Psychiatry. 2014. PMID: 24560582 Free PMC article. - MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons.
Hagenston AM, Fitzpatrick JS, Yeckel MF. Hagenston AM, et al. Cereb Cortex. 2008 Feb;18(2):407-23. doi: 10.1093/cercor/bhm075. Epub 2007 Jun 14. Cereb Cortex. 2008. PMID: 17573372 Free PMC article. - Gq-Coupled Muscarinic Receptor Enhancement of KCNQ2/3 Channels and Activation of TRPC Channels in Multimodal Control of Excitability in Dentate Gyrus Granule Cells.
Carver CM, Shapiro MS. Carver CM, et al. J Neurosci. 2019 Feb 27;39(9):1566-1587. doi: 10.1523/JNEUROSCI.1781-18.2018. Epub 2018 Dec 28. J Neurosci. 2019. PMID: 30593498 Free PMC article. - Small-conductance Ca2+-activated K+ channels: form and function.
Adelman JP, Maylie J, Sah P. Adelman JP, et al. Annu Rev Physiol. 2012;74:245-69. doi: 10.1146/annurev-physiol-020911-153336. Epub 2011 Sep 19. Annu Rev Physiol. 2012. PMID: 21942705 Review. - Control of neuronal excitability by Group I metabotropic glutamate receptors.
Correa AMB, Guimarães JDS, Dos Santos E Alhadas E, Kushmerick C. Correa AMB, et al. Biophys Rev. 2017 Oct;9(5):835-845. doi: 10.1007/s12551-017-0301-7. Epub 2017 Aug 23. Biophys Rev. 2017. PMID: 28836161 Free PMC article. Review.
Cited by
- Group 1 metabotropic glutamate receptor 5 is involved in synaptically-induced Ca2+-spikes and cell death in cultured rat hippocampal neurons.
Yang JS, Jeon S, Jang HJ, Yoon SH. Yang JS, et al. Korean J Physiol Pharmacol. 2022 Nov 1;26(6):531-540. doi: 10.4196/kjpp.2022.26.6.531. Korean J Physiol Pharmacol. 2022. PMID: 36302627 Free PMC article. - Costimulation of AMPA and metabotropic glutamate receptors underlies phospholipase C activation by glutamate in hippocampus.
Kim HH, Lee KH, Lee D, Han YE, Lee SH, Sohn JW, Ho WK. Kim HH, et al. J Neurosci. 2015 Apr 22;35(16):6401-12. doi: 10.1523/JNEUROSCI.4208-14.2015. J Neurosci. 2015. PMID: 25904792 Free PMC article. - K(Ca)2 and k(ca)3 channels in learning and memory processes, and neurodegeneration.
Kuiper EF, Nelemans A, Luiten P, Nijholt I, Dolga A, Eisel U. Kuiper EF, et al. Front Pharmacol. 2012 Jun 11;3:107. doi: 10.3389/fphar.2012.00107. eCollection 2012. Front Pharmacol. 2012. PMID: 22701424 Free PMC article. - Activation of TRPC1 Channel by Metabotropic Glutamate Receptor mGluR5 Modulates Synaptic Plasticity and Spatial Working Memory.
Lepannetier S, Gualdani R, Tempesta S, Schakman O, Seghers F, Kreis A, Yerna X, Slimi A, de Clippele M, Tajeddine N, Voets T, Bon RS, Beech DJ, Tissir F, Gailly P. Lepannetier S, et al. Front Cell Neurosci. 2018 Sep 14;12:318. doi: 10.3389/fncel.2018.00318. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30271326 Free PMC article. - NMDA receptor-dependent synaptic activation of TRPC channels in olfactory bulb granule cells.
Stroh O, Freichel M, Kretz O, Birnbaumer L, Hartmann J, Egger V. Stroh O, et al. J Neurosci. 2012 Apr 25;32(17):5737-46. doi: 10.1523/JNEUROSCI.3753-11.2012. J Neurosci. 2012. PMID: 22539836 Free PMC article.
References
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. - PubMed
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. - PubMed
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1RL1AA017536-01/AA/NIAAA NIH HHS/United States
- R01 MH067830/MH/NIMH NIH HHS/United States
- P50 MH068789/MH/NIMH NIH HHS/United States
- RL1 AA017536/AA/NIAAA NIH HHS/United States
- R01-MH067830/MH/NIMH NIH HHS/United States
- P50-MH068789/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous