A TRPA1-dependent mechanism for the pungent sensation of weak acids - PubMed (original) (raw)
A TRPA1-dependent mechanism for the pungent sensation of weak acids
Yuanyuan Y Wang et al. J Gen Physiol. 2011 Jun.
Abstract
Acetic acid produces an irritating sensation that can be attributed to activation of nociceptors within the trigeminal ganglion that innervate the nasal or oral cavities. These sensory neurons sense a diverse array of noxious agents in the environment, allowing animals to actively avoid tissue damage. Although receptor mechanisms have been identified for many noxious chemicals, the mechanisms by which animals detect weak acids, such as acetic acid, are less well understood. Weak acids are only partially dissociated at neutral pH and, as such, some can cross the cell membrane, acidifying the cell cytosol. The nociceptor ion channel TRPA1 is activated by CO(2), through gating of the channel by intracellular protons, making it a candidate to more generally mediate sensory responses to weak acids. To test this possibility, we measured responses to weak acids from heterologously expressed TRPA1 channels and trigeminal neurons with patch clamp recording and Ca(2+) microfluorometry. Our results show that heterologously expressed TRPA1 currents can be induced by a series of weak organic acids, including acetic, propionic, formic, and lactic acid, but not by strong acids. Notably, the degree of channel activation was predicted by the degree of intracellular acidification produced by each acid, suggesting that intracellular protons are the proximate stimulus that gates the channel. Responses to weak acids produced a Ca(2+)-independent inactivation that precluded further activation by weak acids or reactive chemicals, whereas preactivation by reactive electrophiles sensitized TRPA1 channels to weak acids. Importantly, responses of trigeminal neurons to weak acids were highly overrepresented in the subpopulation of TRPA1-expressing neurons and were severely reduced in neurons from TRPA1 knockout mice. We conclude that TRPA1 is a general sensor for weak acids that produce intracellular acidification and suggest that it functions within the pain pathway to mediate sensitivity to cellular acidosis.
Figures
Figure 1.
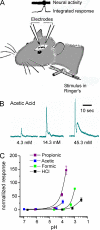
Responses of the trigeminal nerve to acetic acid cannot be attributed to changes in extracellular pH. (A) Diagram showing the method of stimulus delivery and position of the recording electrode. (B) Integrated nerve responses to acetic acid applied at increasing concentrations as indicated. (C) Relationship between the magnitude of the nerve response and the extracellular pH for each acid tested. Responses were normalized to the response to cyclohexanone applied immediately before and after exposure to each acid. Data represent the mean ± SEM.
Figure 2.
Acetic acid activates TRPA1 but not TRPV1. Whole cell currents evoked in response to acids from untransfected HEK-293 cells or HEK-293 cells transfected with YFP-rTRPA1 or rTRPV1 as indicated. (A) 10 mM of acetic acid (HOAc) titrated to pH 5, but not 10 mM MES, pH 5, or 10 mM HEPES, pH 5, activated a large, outwardly rectifying current in TRPA1-expressing cells (right). Current–voltage relationship obtained from ramp depolarization (1V/s) at the time indicated shows reversal of the current near 0 mV and mild outward rectification. Small currents were evoked in untransfected cells in response to pH 5.0 solution buffered with MES, HEPES, or acetic acid (left). (B) Average data from experiments as in A show that the activation of TRPA1 by acetic acid is conserved in humans and rodents. Significance (compared with activation of untransfected cells by acetic acid) was determined with the one-tailed Student’s t test (n = 4–7). Representative traces for responses from mouse or human transfected cells are shown on the right. (C) 10 mM MES, pH 5.0, activated a large TRPV1 current, which was inhibited by subsequent application of 10 mM of acetic acid, pH 5. (D) Average data (n = 10) from experiments as in C, where current decay was measured during two 10-s windows immediately before and after acetic acid application. Significance was determined by the Wilcoxon signed-rank test. Average data are represented by the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 3.
TRPA1 is activated by weak acids that acidify the cell cytosol. (A) Changes in emission from the pH-sensitive fluorescent probe carboxy-DFFDA in HEK-293 cells in response to acetic acid at varying concentrations (0, 0.5, 2, 10, and 100 mM at pH 5) and pH (10 mM; pH 5, 6, and 7) and to a panel of carboxylic acids (10 or 100 mM; pH 5). A representative experiment is shown on the left. (B) Proposed model for how acetic acid might activate TRPA1. (C) Currents evoked in HEK-293 cells expressing TRPA1 (peak magnitude at + 80 mV) in response to acetic acid at varying concentrations and pH, and to other carboxylic acids as indicated. Same color scheme as in A. Data for 0 and 10 mM of acetic acid, pH 5, were reproduced from Fig. 2 B. Representative traces of current activation by 100 mM of lactic acid (LA), pH 5, and 10 mM PA, pH 5, are shown on the left. Inset shows the structures of the acids. (D) The magnitude of the TRPA1 current plotted as a function of the change in fluorescence of the pH-sensitive dye carboxy-DFFDA. Colors correspond to the scheme in A and B. The correlation between the change in fluorescence and the magnitude of the TRPA1 current suggests that intracellular pH is the proximate stimulus that gates TRPA1 in response to extracellularly applied weak acids. Data are represented by the mean ± SEM.
Figure 4.
TRPA1 channels are activated by intracellular protons. (A) Channel activity from a TRPA1-expressing HEK-293 cell in cell-attached patch clamp (Vm = −80 mV) in response to the addition of 10 mM of acetic acid at pH 5.0 outside the patch. (B) Openings of single channels are shown on an expanded time scale from before, during, and after the application of acetic acid as indicated. Similar results were obtained in six out of six patches. (C) Channel activity in an inside-out patch from a HEK-293 cell transfected with TRPA1 (Vm = −80 mV) in response to cytoplasmic delivery of acetate anions or protons (pH 5.5; 1 mM polyP3 was added to all solutions to retain channel activity; Kim and Cavanaugh, 2007). Summary data represent the mean ± SEM. **, P < 0.01 (two-tailed Student’s t test).
Figure 5.
Acid and reactive compounds act on different sites of TRPA1. Whole cell currents evoked in HEK-293 cells transfected with wild-type TRPA1 or cysteine mutant C622S. (A and B) 100 µM Cin strongly activated wild-type TRPA1 but only weakly activated the TRPA1 mutant C622S. The subsequent addition of 2 mM Ca2+ induced no further activation and promoted rapid inactivation of both wild-type and C622S currents. In contrast, 10 mM of acetic acid, pH 5.0, strongly activated both wild-type TRPA1- and C622S-mutant channels. (C) Average peak current amplitude measured at +80 mV from experiments as in A and B. Comparison between wild type and C622S was with the two-tailed Student’s t test. Data are represented by the mean ± SEM. **, P < 0.01.
Figure 6.
Responses of TRPA1 to weak acids self-desensitize and cross-desensitize responses to Cin. Whole cell currents evoked in HEK-293 cells expressing TRPA1. (A) TRPA1 currents evoked in response to 10 mM of acetic acid, pH 5, decayed after activation and could not be evoked again by acetic acid, indicating that the channels had entered an inactivated state. (B) After inactivation by 10 mM of acetic acid, pH 5, TRPA1 currents could not be activated by 100 µM Cin, indicating that acetic acid could cross-desensitize responses to Cin. (C) 100 µM Cin elicited large currents in TRPA1-expressing cells that were not preexposed to acetic acid. Currents were recorded in the absence of extracellular Ca2+. Average data are shown below each representative trace. Data are represented by the mean ± SEM. Significance was determined with the two-tailed paired (A; comparison between responses to the first and second application of acetic acid) or unpaired (B and C; comparison between responses to Cin with or without preexposure to acetic acid) Student’s t test. *, P < 0.05; ***, P < 0.001.
Figure 7.
Lactic acid and MO act in synergy to activate TRPA1. (A) 20 mM lactic acid, pH 5, activated small TRPA1 currents in TRPA1-expressing HEK-293 cells (top). The addition of extracellular Ca2+ produced no enhancement of activation by lactic acid (bottom). (B) Preexposure to MO greatly increased TRPA1 current activation in response to lactic acid. (C) Average data from experiments as in A and B. Data are represented by the mean ± SEM. Significance was determined with the two-tailed Student’s t test. *, P < 0.05; **, P < 0.01.
Figure 8.
PA activates TRPA1 in Ca2+ imaging. Agonist-induced elevation of intracellular Ca2+ in HEK-293 cells transfected with TRPV1 or TRPA1. (A) TRPA1-expressing HEK cells responded to both 100 mM PA, pH 6.5, and 100 µM Cin. (B) TRPV1-expressing HEK cells responded only to 1 µM Cap and showed nonspecific responses to PA. Scatter plot shows the amplitude of the responses to PA as a function of the responses to Cin (A) or Cap (B). Average responses to PA and Cin (A) or PA and Cap (B) are shown in the bar graph. Data are represented by the mean ± SEM. Significance was determined with the two-tailed Student’s t test. ***, P < 0.001.
Figure 9.
Responses of TG neurons to weak acids are TRPA1 depedent. (A) Elevation of intracellular Ca2+ in sensory neurons isolated from the TG of wild-type mice in response to weak acids and other agonists. Traces illustrate the different types of responses observed: cells responsive to PA, Cin, and Cap (but not to 10 mM HEPES, pH 6.5) are shown with green, blue, and cyan traces; a cell responsive to Cap, but not to Cin or PA, is shown in red; and a cell that responded to none of the agonists is shown in black. (B) TG neurons from TRPA1 knockout mice showed only reduced sensitivity to PA and Cin but retained normal responses to Cap (orange and red traces). Venn diagrams show the aggregate results from all experiments. Numbers represent a total count of the responsive cells to each agonist, and overlap and size of each circle are drawn to scale. (C) Summary data from experiments as in A and B showing the magnitude of the PA response as a function of the Cin response in the same cell. Note that the PA response was positively correlated with the magnitude of the Cin response in cells from wild-type mice (r = 0.52 and P < 0.0001). (D) The percentage of different populations of TG neurons in cultures from wild-type and TRPA1 knockout mice.
Comment in
- Weakly acidic, but strongly irritating: TRPA1 and the activation of nociceptors by cytoplasmic acidification.
Garrity PA. Garrity PA. J Gen Physiol. 2011 Jun;137(6):489-91. doi: 10.1085/jgp.201110657. Epub 2011 May 16. J Gen Physiol. 2011. PMID: 21576374 Free PMC article. No abstract available.
Similar articles
- The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6.
de la Roche J, Eberhardt MJ, Klinger AB, Stanslowsky N, Wegner F, Koppert W, Reeh PW, Lampert A, Fischer MJ, Leffler A. de la Roche J, et al. J Biol Chem. 2013 Jul 12;288(28):20280-92. doi: 10.1074/jbc.M113.479337. Epub 2013 May 24. J Biol Chem. 2013. PMID: 23709225 Free PMC article. - Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain.
Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Dai Y, et al. J Clin Invest. 2007 Jul;117(7):1979-87. doi: 10.1172/JCI30951. J Clin Invest. 2007. PMID: 17571167 Free PMC article. - Primary alcohols activate human TRPA1 channel in a carbon chain length-dependent manner.
Komatsu T, Uchida K, Fujita F, Zhou Y, Tominaga M. Komatsu T, et al. Pflugers Arch. 2012 Apr;463(4):549-59. doi: 10.1007/s00424-011-1069-4. Epub 2012 Jan 6. Pflugers Arch. 2012. PMID: 22222967 - TRPA1.
Zygmunt PM, Högestätt ED. Zygmunt PM, et al. Handb Exp Pharmacol. 2014;222:583-630. doi: 10.1007/978-3-642-54215-2_23. Handb Exp Pharmacol. 2014. PMID: 24756722 Review. - Irritating channels: the case of TRPA1.
Nilius B, Prenen J, Owsianik G. Nilius B, et al. J Physiol. 2011 Apr 1;589(Pt 7):1543-9. doi: 10.1113/jphysiol.2010.200717. Epub 2010 Nov 15. J Physiol. 2011. PMID: 21078588 Free PMC article. Review.
Cited by
- Functionally active TRPA1 ion channel is downregulated in peptidergic neurons of the Edinger-Westphal nucleus upon acute alcohol exposure.
Al-Omari A, Kecskés M, Gaszner B, Biró-Sütő T, Fazekas B, Berta G, Kuzma M, Pintér E, Kormos V. Al-Omari A, et al. Front Cell Dev Biol. 2023 Jan 10;10:1046559. doi: 10.3389/fcell.2022.1046559. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36704197 Free PMC article. - Chemosensory Contributions of E-Cigarette Additives on Nicotine Use.
Johnson NL, Patten T, Ma M, De Biasi M, Wesson DW. Johnson NL, et al. Front Neurosci. 2022 Jul 19;16:893587. doi: 10.3389/fnins.2022.893587. eCollection 2022. Front Neurosci. 2022. PMID: 35928010 Free PMC article. Review. - Redefining our menu: communications and reviews.
Pugh EN Jr. Pugh EN Jr. J Gen Physiol. 2011 Aug;138(2):131. doi: 10.1085/jgp.201110688. J Gen Physiol. 2011. PMID: 21788608 Free PMC article. No abstract available. - Intracellular proton-mediated activation of TRPV3 channels accounts for the exfoliation effect of α-hydroxyl acids on keratinocytes.
Cao X, Yang F, Zheng J, Wang K. Cao X, et al. J Biol Chem. 2012 Jul 27;287(31):25905-16. doi: 10.1074/jbc.M112.364869. Epub 2012 Jun 7. J Biol Chem. 2012. PMID: 22679014 Free PMC article. - Diesel exhaust modulates ozone-induced lung function decrements in healthy human volunteers.
Madden MC, Stevens T, Case M, Schmitt M, Diaz-Sanchez D, Bassett M, Montilla TS, Berntsen J, Devlin RB. Madden MC, et al. Part Fibre Toxicol. 2014 Sep 2;11:37. doi: 10.1186/s12989-014-0037-5. Part Fibre Toxicol. 2014. PMID: 25178924 Free PMC article. Clinical Trial.
References
- Bryant B.P., Moore P.A. 1995. Factors affecting the sensitivity of the lingual trigeminal nerve to acids. Am. J. Physiol. 268:R58–R65 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC004564/DC/NIDCD NIH HHS/United States
- R01 DC004564-12/DC/NIDCD NIH HHS/United States
- R01 DC004564-13/DC/NIDCD NIH HHS/United States
- DC004564/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous