A motif unique to the human DEAD-box protein DDX3 is important for nucleic acid binding, ATP hydrolysis, RNA/DNA unwinding and HIV-1 replication - PubMed (original) (raw)
A motif unique to the human DEAD-box protein DDX3 is important for nucleic acid binding, ATP hydrolysis, RNA/DNA unwinding and HIV-1 replication
Anna Garbelli et al. PLoS One. 2011.
Abstract
DEAD-box proteins are enzymes endowed with nucleic acid-dependent ATPase, RNA translocase and unwinding activities. The human DEAD-box protein DDX3 has been shown to play important roles in tumor proliferation and viral infections. In particular, DDX3 has been identified as an essential cofactor for HIV-1 replication. Here we characterized a set of DDX3 mutants biochemically with respect to nucleic acid binding, ATPase and helicase activity. In particular, we addressed the functional role of a unique insertion between motifs I and Ia of DDX3 and provide evidence for its implication in nucleic acid binding and HIV-1 replication. We show that human DDX3 lacking this domain binds HIV-1 RNA with lower affinity. Furthermore, a specific peptide ligand for this insertion selected by phage display interferes with HIV-1 replication after transduction into HelaP4 cells. Besides broadening our understanding of the structure-function relationships of this important protein, our results identify a specific domain of DDX3 which may be suited as target for antiviral drugs designed to inhibit cellular cofactors for HIV-1 replication.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
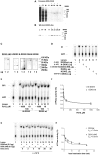
Figure 1. Human DDX3 has a unique insertion between motifs I and Ia.
A. Multiple sequence alignment of the region between the Q-box and motif Ia of human DDX3 (accession n. NP001347) with S. cerevisiae Ded1p (accession n. P06634), S. cerevisiae Dbp1 (accession n. P2478), S. pombe Ded1 (accession n. O13370), M. musculus Pl10 (accession n. NP149068) and X. laevis An3 (accession n. NP001095245) proteins. Identical/equivalent amino acids are shaded. Residues of the conserved motifs are in bold. The DDX3 specific insertion is indicated with a black box. Multiple alignments were performed with the program ClustalW2 (
). B. Schematic representation of the length of the insertion sequences between motifs I and Ia of DDX3 and different DEAD-box proteins. The number of amino acids in each insertion is indicated. C. Multiple sequence alignment of the region between motif I and Ia of DDX3 and different DEAD-box proteins. Identical amino acids are shaded in dark grey, equivalent amino acids are shaded in light grey. Basic amino acids in the DDX3 insertion are in bold. Multiple alignments were performed with the program ClustalW2 (
). D. Schematic representation of the different mutants used in this study. The conserved motifs are named after their consensus sequence and represented as boxes. The name of the different mutants is on the left of the panel, while the corresponding amino acids are indicated on the right side, along with the corresponding biochemical properties revealed in this study. +, 100% (basal activity); ++, +++, stimulation of basal activity; +/−, reduced basal activity; −, no activity. Boxed residues in motifs I and II of the K230E and DADA mutants identify the single amino acid changes.
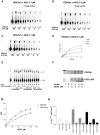
Figure 2. Purification of recombinant human DDX3.
A. Coomassie staining of SDS-PAGE of the full length human DDX3 containing fractions eluted from the hydroxyhapatite column. L, loading; FT, flow-through; W, wash. Molecular weight is indicated on the right side. Fraction numbers are on top. Asterisks indicate the fractions used for the experiments. B. Western blot analysis of the fractions shown in panel A with anti-human DDX3 polyclonal antibodies. C. Coomassie staining of SDS-PAGE of the final purified preparation of all the recombinant DDX3 proteins. D. ATPase activity of all the recombinant DDX3 proteins. Reactions were performed as described in Material and Methods in the absence of nucleic acids. Unreacted substrate (ATP) was separated from the product (phosphate, Pi) by thin layer chromatography. The length (amino acids) of each protein is indicated in brackets. E. ATPase activity of N-DDX3 (lanes 2–6) or DDX3wt (lanes 7–11) in the absence (lanes 2 and 7) or in the presence of increasing amounts of the DDX3 inhibitor FE15. Lane 1, control without enzyme. F. Dose-response curves for the inhibition by FE15 of the ATPase activity of DDX3wt (circles) or N-DDX3 (triangles). Values are means of three independent determinations. Error bars are ±S.D. G. Representative time course experiment of the ATPase activity of DDX3wt (even lanes) or DDXΔINS (odd lanes) at 70°C. Lane 1, control without enzyme. H. Activity decay curves for the ATP hydrolysis by DDX3wt (circles) or DDX3ΔINS (triangles). Curves were fitted to a simple exponential decay model. Values are means of three independent determinations. Error bars are ±S.D.
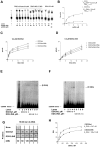
Figure 3. Characterization of the ATPase activity of recombinant human DDX3.
Reactions were performed as described in Material and Methods. A. Product analysis of a representative experiment for the ATPase reaction catalyzed by 0.1 µM full length DDX3 in the presence of 10 µM ss RNA. B. As in panel A, but in the presence of 10 µM ss DNA. C. As in panel A, but in the absence of nucleic acids. D. Variation of the initial velocities of the reaction as a function of ATP concentrations, in the absence (triangles) or in the presence of 10 µM ss DNA (circles) or 10 µM ss RNA (squares). Data were fitted to Eq.(1) (see Materials and Methods). Values are the means of three independent determinations. Error bars are ±SD. E. Product analysis of the ATPase reaction catalyzed by increasing amounts of the DDX3ΔINS mutant in the absence (lanes 2–5) or in the presence of of 25 µM ss DNA (lanes 7–10) or ssRNA (lanes 12–15). Lanes 1, 6 and 11, control reactions in the absence of enzyme. F. [α-33P] ATP was UV crosslinked to increasing amounts of DDX3wt (upper panel) or ΔINS mutant (lower panel). Radioactive proteins were resolved on SDS-PAGE and revealed by phosphoImaging. G. Binding of DDX3 to ATP, as revealed by UV-crosslinking. Data were fitted to Eq.(5) (see Materials and Methods). Values are the means of three independent determinations. Error bars are ±SD. H. Comparison of the catalytic efficiencies (kcat/Km) for ATP hydrolysis of DDX3wt (aa 1–662), and the N-DDX3 (aa 1–427), DDX3ΔINS and I-DDX3 (aa 160–427) mutants, in the absence (white bars) or in the presence of ssDNA (grey bars) or ssRNA (black bars). Determination of the kinetic constants kcat and Km was performed as described in Materials and Methods. Values represent the means between two independent estimates of the kcat/Km values from two sets of experiments. Error bars represent ±SD.
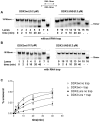
Figure 4. The specific insertion of human DDX3 is important for nucleic-acid stimulation of ATPase activity.
Reactions were performed as described in Material and Methods. A. Product analysis for the ATPase reaction catalyzed by the DDX3wt (lanes 2–6) and the DDX3ΔINS (lanes 7–11) mutant proteins in the absence (lanes 6, 11) or in the presence of increasing concentrations of ssDNA. Lane 1, control reaction without enzyme. Reactions with the full length DDX1 protein (lanes 12–14) in the absence (lane 12) or in the presence of a fixed amount of DNA (lane 13) or RNA (lane 14), were included for comparison. B. Variation of the increase in the ATPase reaction rate (Δv) as a function of the ssDNA concentration in the presence of DDX3wt (circles) or DDX3ΔINS (triangles). The Δv values were derived as described in Materials and Methods. Data were fitted to Eq.(2) (see Materials and Methods). Values are the means of three independent determinations. Error bars are ±SD. C. Progress curves for the product formation during the ATPase reaction catalyzed by 0.2 µM of DDX3wt as a function of time, in the absence (circles) or in the presence of 10 µM ssRNA (triangles) or 10 µM ssDNA (squares). Data were fitted to Eq. (3) (see Materials and Methods). Values are the means of three independent determinations. Error bars are ±SD. D. As in panel C, but in the presence of 0.2 µM of the DDX3ΔINS mutant. E. Increasing amounts of DDX3wt (second row) or DDX3ΔINS mutant (third row), were incubated with a fixed concentration of (6-FAM)-5′-labelled ssDNA oligonucleotide. Nitrocellulose filter bound protein-DNA complexes were revealed by laser scanning. First row, control in the absence of proteins. F. Binding of DDX3 to ssDNA, as revealed by filter-binding assays. Data were fitted to Eq.(5) (see Materials and Methods). Values are the means of three independent determinations. Error bars are ±SD. G. Increasing amounts of DDX3wt (lanes 2–5) or DDX3ΔINS (lanes 6–9) were incubated in the presence of a (6-FAM)-5′-labelled ss RNA oligonucleotide. Enzyme-RNA ([E:RNA]) complexes were resolved by non denaturing PAGE and visualized by laser scanning. Lane 1, oligonucleotide alone.
Figure 5. The specific insertion of human DDX3 is important for RNA unwinding.
Reactions were performed as described in Materials and Methods. A. Representative RNA unwinding assay for DDX3wt, or the DDX3ΔINS mutant. Lanes 8, 16, boiled controls. B. As in A, but with the addition of a 50- fold molar excess of a r(A)40mer RNA trap ss oligonucleotide. C. Quantitative analysis of the RNA unwinding reaction catalyzed by DDX3wt and the DDX3ΔINS mutant. Values are the means of three independent determinations. Error bars are ±SD.
Figure 6. Human DDX3 can act as a DNA helicase.
Experiments were performed as described in Materials and Methods. The 18-mer oligonucleotide was fluorescently labelled at its 5′-end in all the experiments. The position of the unreacted substrate (18/38-mer) and of the released strand 18-mer are indicated on the left side of each panel. A. Strand-displacement reactions catalyzed by increasing amounts of DDX3wt in the presence of the different partially dsDNA substrates: 18/[5′-3′]-38 mer (lanes 1–5); 18/[3′]-38 mer (lanes 6–10); 18/[5′]-38 mer (lanes 11–15). Lanes 1, 6, 11: control reactions incubated in the absence of enzyme. Lanes 2, 7, 12: reaction mix with no enzyme loaded on gel without incubation. Lane 16: boiled substrate. B. Strand displacement activity of increasing amounts of the DDX1 (lanes 3–5) or DDX3ΔINS (lanes 7–9) proteins on the 18/[5′-3′]-38 mer substrate. Lanes 2, 6, control reactions incubated in the absence of enzyme; lane 1, boiled substrate. C. Sequence alignment of human DDX3, S. cerevisiae Dpb9, human DDX1, D. melanogaster Vasa and human Dpb5, showing the structural motifs Ia, Ib and IV. Residues known to mediate specific 2′-OH ribose contacts in the Vasa crystal structure are highlighted. The corresponding helicase substrates for each enzyme are indicated on the right side of the panel.
Figure 7. The DDX3 insert is functionally implicated in the HIV-1 cofactor activity of DDX3.
A. HIV genomic RNA pull down experiments with purified DDX3wt and DDX3ΔINS proteins. Bound HIV RNA was detected by RT-PCR with _gag_-specific primers in three independent experiments (lanes 1 and 2; lanes 3 and 4; lanes 8 and 9). Lanes 6 and 7 correspond to RT-PCR fragments of input viral RNA and PCR of the HIV-1 pLai plasmid, respectively; lane 5 is the negative PCR control. B. Peptide DDX3-INS1 selected with the DDX3 insertion by phage display shows homology to XPO1. The crystal structure of XPO1 (DOI:10.2210/pdb3gb8/pdb) is shown and amino acids identical to the peptide sequence are represented as spheres. The corresponding positions are indicated on the right side of the panel, along with the conservative substitution V408L. C. Antiviral activity of the selected peptide DDX3-INS1 fused to a protein transduction domain. Peptides DDX3-INS1 and a control peptide were transduced into HelaP4 cells after infection with HIV-1Lai and virus in supernatants was quantified on Tzm-bl cells by luminometry after 44 hours. Bars represent the mean of 3 independent experiments and error bars indicate SD. For the control peptide, one representative experiment is shown.
Similar articles
- DDX3 DEAD-Box RNA helicase inhibits hepatitis B virus reverse transcription by incorporation into nucleocapsids.
Wang H, Kim S, Ryu WS. Wang H, et al. J Virol. 2009 Jun;83(11):5815-24. doi: 10.1128/JVI.00011-09. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297497 Free PMC article. - A Survey of DDX21 Activity During Rev/RRE Complex Formation.
Hammond JA, Zhou L, Lamichhane R, Chu HY, Millar DP, Gerace L, Williamson JR. Hammond JA, et al. J Mol Biol. 2018 Feb 16;430(4):537-553. doi: 10.1016/j.jmb.2017.06.023. Epub 2017 Jul 10. J Mol Biol. 2018. PMID: 28705764 Free PMC article. - DEAD-box RNA Helicase DDX3: Functional Properties and Development of DDX3 Inhibitors as Antiviral and Anticancer Drugs.
Kukhanova MK, Karpenko IL, Ivanov AV. Kukhanova MK, et al. Molecules. 2020 Feb 24;25(4):1015. doi: 10.3390/molecules25041015. Molecules. 2020. PMID: 32102413 Free PMC article. Review. - Autoinhibitory Interdomain Interactions and Subfamily-specific Extensions Redefine the Catalytic Core of the Human DEAD-box Protein DDX3.
Floor SN, Condon KJ, Sharma D, Jankowsky E, Doudna JA. Floor SN, et al. J Biol Chem. 2016 Jan 29;291(5):2412-21. doi: 10.1074/jbc.M115.700625. Epub 2015 Nov 23. J Biol Chem. 2016. PMID: 26598523 Free PMC article. - RNA helicase DDX3: at the crossroad of viral replication and antiviral immunity.
Valiente-Echeverría F, Hermoso MA, Soto-Rifo R. Valiente-Echeverría F, et al. Rev Med Virol. 2015 Sep;25(5):286-99. doi: 10.1002/rmv.1845. Epub 2015 Jul 14. Rev Med Virol. 2015. PMID: 26174373 Review.
Cited by
- The human DEAD-box helicase DDX3X as a regulator of mRNA translation.
Ryan CS, Schröder M. Ryan CS, et al. Front Cell Dev Biol. 2022 Oct 25;10:1033684. doi: 10.3389/fcell.2022.1033684. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393867 Free PMC article. Review. - NZ51, a ring-expanded nucleoside analog, inhibits motility and viability of breast cancer cells by targeting the RNA helicase DDX3.
Xie M, Vesuna F, Botlagunta M, Bol GM, Irving A, Bergman Y, Hosmane RS, Kato Y, Winnard PT Jr, Raman V. Xie M, et al. Oncotarget. 2015 Oct 6;6(30):29901-13. doi: 10.18632/oncotarget.4898. Oncotarget. 2015. PMID: 26337079 Free PMC article. - The Continuing Evolution of HIV-1 Therapy: Identification and Development of Novel Antiretroviral Agents Targeting Viral and Cellular Targets.
Hartman TL, Buckheit RW Jr. Hartman TL, et al. Mol Biol Int. 2012;2012:401965. doi: 10.1155/2012/401965. Epub 2012 Jul 10. Mol Biol Int. 2012. PMID: 22848825 Free PMC article. - Chemoproteomic profiling unveils binding and functional diversity of endogenous proteins that interact with endogenous triplex DNA.
Xu H, Ye J, Zhang KX, Hu Q, Cui T, Tong C, Wang M, Geng H, Shui KM, Sun Y, Wang J, Hou X, Zhang K, Xie R, Yin Y, Chen N, Chen JY. Xu H, et al. Nat Chem. 2024 Nov;16(11):1811-1821. doi: 10.1038/s41557-024-01609-7. Epub 2024 Sep 2. Nat Chem. 2024. PMID: 39223307 - A novel de novo DDX3X missense variant in a female with brachycephaly and intellectual disability: a case report.
Moresco G, Costanza J, Santaniello C, Rondinone O, Grilli F, Prada E, Orcesi S, Coro I, Pichiecchio A, Marchisio P, Miozzo M, Fontana L, Milani D. Moresco G, et al. Ital J Pediatr. 2021 Mar 31;47(1):81. doi: 10.1186/s13052-021-01033-4. Ital J Pediatr. 2021. PMID: 33789733 Free PMC article.
References
- Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. - PubMed
- Abdelhaleem M, Maltais L, Wain H. The human DDX and DHX gene families of putative RNA helicases. Genomics. 2003;81:618–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases