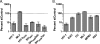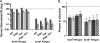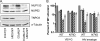The requirement for nucleoporin NUP153 during human immunodeficiency virus type 1 infection is determined by the viral capsid - PubMed (original) (raw)
The requirement for nucleoporin NUP153 during human immunodeficiency virus type 1 infection is determined by the viral capsid
Kenneth A Matreyek et al. J Virol. 2011 Aug.
Abstract
Lentiviruses likely infect nondividing cells by commandeering host nuclear transport factors to facilitate the passage of their preintegration complexes (PICs) through nuclear pore complexes (NPCs) within nuclear envelopes. Genome-wide small interfering RNA screens previously identified karyopherin β transportin-3 (TNPO3) and NPC component nucleoporin 153 (NUP153) as being important for infection by human immunodeficiency virus type 1 (HIV-1). The knockdown of either protein significantly inhibited HIV-1 infectivity, while infection by the gammaretrovirus Moloney murine leukemia virus (MLV) was unaffected. Here, we establish that primate lentiviruses are particularly sensitive to NUP153 knockdown and investigate HIV-1-encoded elements that contribute to this dependency. Mutants lacking functional Vpr or the central DNA flap remained sensitive to NUP153 depletion, while MLV/HIV-1 chimera viruses carrying MLV matrix, capsid, or integrase became less sensitive when the latter two elements were substituted. Two capsid missense mutant viruses, N74D and P90A, were largely insensitive to NUP153 depletion, as was wild-type HIV-1 when cyclophilin A was depleted simultaneously or when infection was conducted in the presence of cyclosporine A. The codepletion of NUP153 and TNPO3 yielded synergistic effects that outweighed those calculated based on individual knockdowns, indicating potential interdependent roles for these factors during HIV-1 infection. Quantitative PCR revealed normal levels of late reverse transcripts, a moderate reduction of 2-long terminal repeat (2-LTR) circles, and a relatively large reduction in integrated proviruses upon NUP153 knockdown. These results suggest that capsid, likely by the qualities of its uncoating, determines whether HIV-1 requires cellular NUP153 for PIC nuclear import.
Figures
Fig. 1.
NUP153 expression and HIV-1 infection. (A) Twofold dilutions of a whole-cell extract from control cells (lanes 1 to 4) compared to extracts from NUP153-depleted cells (lanes 5 and 6). NUP62 cross-reacted with the utilized anti-NUP153 antibody. (B) Percent infectivity of GFP reporter viruses in HeLa cells transfected with siNUP153#1 (dark gray) or siNUP153#2 (light gray) compared to control cells. Results are averages from three experiments, each performed in triplicate; error bars denote 95% confidence intervals. (C) HEK293T cells transfected with siControl or siNUP153#1 were retransfected with either control DNA (pUC19), empty IRES-dsRed-Express vector, or the vector expressing siRNA-resistant NUP153 protein. (D) Cells in panel C were gated for dim dsRed-Express expression, and the infectivities of GFP reporter viruses were normalized to those of cells transfected with the empty vector. Solid and hatched bars, cells transfected with siControl and siNUP153#1, respectively; dark and light gray bars, cells transfected with empty and NUP153 expression vectors, respectively. The results are averages from four experiments performed in duplicate, with error bars denoting 95% confidence intervals.
Fig. 2.
Retroviral susceptibilities to NUP153 knockdown. HeLa cells transfected with siNUP153#1 or siControl were infected with GFP reporter viruses specific to primate lentiviruses (A) or different types of retroviruses (B). Results are averages from at least three experiments performed in triplicate, with error bars denoting 95% confidence intervals.
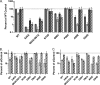
Fig. 3.
NUP153 dependency during HIV-1 infection is independent of Vpr and the central DNA flap. (A) Viral infectivities were normalized to the level obtained with 5 × 106 RTcpm of WT virus (set to 100%). Dark gray, siControl; light gray, siNUP153#1. (B) Regraph of panel A results, with infectivities in knockdown cells expressed as percentages of control cells, which were set at 100%. Solid dark gray, WT virus; light gray, DNA flap mutant; hatched bars, Vpr mutant viruses. Results are averages from four experiments performed in duplicate, with error bars denoting 95% confidence intervals.
Fig. 4.
NUP153 dependencies of MLV/HIV-1 chimera viruses. (A) Illustration of constructs tested (not to scale), with major HIV-1 Gag and Pol proteins indicated in gray (NC, nucleocapsid; PR, protease) and MLV proteins in white. (B) Control or knockdown cells were infected with HIV-1LAI, MLV, or HIV-1-derived MLV chimera viruses shown in panel A. Results are averages from three experiments performed in triplicate, with error bars denoting 95% confidence intervals.
Fig. 5.
WT and CA mutant viral infectivities and cyclosporine dependences in control and NUP153 knockdown cells. (A) Control (dark gray) or NUP153 knockdown (light gray) cells were left untreated (solid bars) or were treated with 5 μM cyclosporine (hatched bars) at the time of infection. All samples were normalized to the infectivity of WT virus in untreated control cells, which was set at 100%. The results are averages from three experiments, each performed in duplicate, with error bars denoting 95% confidence intervals. (B) Regraph of panel A results, with infectivities in knockdown cells expressed as percentages of the infectivity of control cells; hatched bars denote infections in the presence of CsA. The results are averages from six experiments performed in duplicate, with error bars denoting 95% confidence intervals. (C) Infectivities in NUP153 knockdown cells compared to that of control cells, with hatched bars denoting cells in which CypA was simultaneously depleted. Results are averages from two experiments performed in duplicate, with error bars denoting 95% confidence intervals.
Fig. 6.
Interdependence of NUP153 and TNPO3 during HIV-1 infection. (A) Whole-cell extracts of control, NUP153-depleted, TNPO3-depleted, and combinatorially depleted cells were blotted with the indicated primary antibodies. (B) Control (dark gray) or NUP153 knockdown (light gray) cells simultaneously depleted for TNPO3 (hatched bars) were infected with 2 × 106 or 2 × 107 RTcpm of VSV-G or HIV-1 envelope pseudotyped viruses, respectively, yielding numbers of RLU in control cells that were within 1 log of each other (not shown). All samples were normalized to the infectivity of the WT virus in control cells, which was set at 100%. White bars show the multiplicative product of infectivity defects exhibited upon individual protein knockdowns, representing the theoretical maximum expected assuming independent function. Results are averages from three experiments, each performed in duplicate, with error bars denoting 95% confidence intervals.
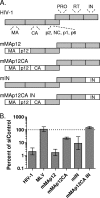
Fig. 7.
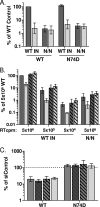
NUP153 dependencies of WT and IN active-site mutant viruses. (A) Relative differences in reporter expression of 5 × 106 RTcpm WT and N/N IN mutant, along with WT sequence or the CA N74D mutation, in the absence (dark gray) or presence (light gray) of 10 μM raltegravir. (B) Relative infectivities of WT and N/N mutant viruses in control (dark gray) or NUP153 knockdown cells (light gray), with WT infectivity (5 × 106 RTcpm) set to 100%. Viruses harbored either WT (solid bars) or N74D (hatched bars) CA. (C) Regraph of panel B results, with infectivities in knockdown cells expressed as percentages of respective control cells; solid, hatched, and boldface hatched bars denote infections with 5 × 106, 5 × 105, and 5 × 104 RTcpm of WT IN virus, respectively, while light gray bars denote infection with N/N virus. Results are averages from five experiments performed in triplicate, with error bars denoting 95% confidence intervals.
Fig. 8.
HIV-1 DNA species formed during acute infection of NUP153 knockdown cells. Viral DNAs were amplified from cells following infection with WT (A, B, and C) or N/N mutant (D and E) virus, with values from NUP153 knockdown cells (gray dashed line) normalized to peak LRT (8 hpi) (A and D), 2-LTR circle (24 hpi) (B and E), and integration (52 hpi) (C) values. (F) Levels of WT and N/N mutant virus infectivities upon NUP153 depletion, expressed as percent siControl-transfected cells (set at 100%). Results are averages from three experiments, with error bars denoting 95% confidence intervals.
Comment in
- Examining the requirements for nucleoporins by HIV-1.
Monette A, Panté N, Mouland AJ. Monette A, et al. Future Microbiol. 2011 Nov;6(11):1247-50. doi: 10.2217/fmb.11.111. Future Microbiol. 2011. PMID: 22082286
Similar articles
- Nup153 Unlocks the Nuclear Pore Complex for HIV-1 Nuclear Translocation in Nondividing Cells.
Buffone C, Martinez-Lopez A, Fricke T, Opp S, Severgnini M, Cifola I, Petiti L, Frabetti S, Skorupka K, Zadrozny KK, Ganser-Pornillos BK, Pornillos O, Di Nunzio F, Diaz-Griffero F. Buffone C, et al. J Virol. 2018 Sep 12;92(19):e00648-18. doi: 10.1128/JVI.00648-18. Print 2018 Oct 1. J Virol. 2018. PMID: 29997211 Free PMC article. - The requirement for cellular transportin 3 (TNPO3 or TRN-SR2) during infection maps to human immunodeficiency virus type 1 capsid and not integrase.
Krishnan L, Matreyek KA, Oztop I, Lee K, Tipper CH, Li X, Dar MJ, Kewalramani VN, Engelman A. Krishnan L, et al. J Virol. 2010 Jan;84(1):397-406. doi: 10.1128/JVI.01899-09. Epub 2009 Oct 21. J Virol. 2010. PMID: 19846519 Free PMC article. - Interplay between HIV entry and transportin-SR2 dependency.
Thys W, De Houwer S, Demeulemeester J, Taltynov O, Vancraenenbroeck R, Gérard M, De Rijck J, Gijsbers R, Christ F, Debyser Z. Thys W, et al. Retrovirology. 2011 Jan 30;8:7. doi: 10.1186/1742-4690-8-7. Retrovirology. 2011. PMID: 21276267 Free PMC article. - Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes.
Matreyek KA, Engelman A. Matreyek KA, et al. Viruses. 2013 Oct 7;5(10):2483-511. doi: 10.3390/v5102483. Viruses. 2013. PMID: 24103892 Free PMC article. Review. - HIV Capsid and Integration Targeting.
Engelman AN. Engelman AN. Viruses. 2021 Jan 18;13(1):125. doi: 10.3390/v13010125. Viruses. 2021. PMID: 33477441 Free PMC article. Review.
Cited by
- Nup153 and Nup98 bind the HIV-1 core and contribute to the early steps of HIV-1 replication.
Di Nunzio F, Fricke T, Miccio A, Valle-Casuso JC, Perez P, Souque P, Rizzi E, Severgnini M, Mavilio F, Charneau P, Diaz-Griffero F. Di Nunzio F, et al. Virology. 2013 May 25;440(1):8-18. doi: 10.1016/j.virol.2013.02.008. Epub 2013 Mar 21. Virology. 2013. PMID: 23523133 Free PMC article. - Human Three Prime Repair Exonuclease 1 Promotes HIV-1 Integration by Preferentially Degrading Unprocessed Viral DNA.
Davids BO, Balasubramaniam M, Sapp N, Prakash P, Ingram S, Li M, Craigie R, Hollis T, Pandhare J, Dash C. Davids BO, et al. J Virol. 2021 Aug 10;95(17):e0055521. doi: 10.1128/JVI.00555-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34105995 Free PMC article. - HIV Capsid Inhibitors Beyond PF74.
McArthur C, Gallazzi F, Quinn TP, Singh K. McArthur C, et al. Diseases. 2019 Oct 30;7(4):56. doi: 10.3390/diseases7040056. Diseases. 2019. PMID: 31671622 Free PMC article. Review. - Nuclear pore heterogeneity influences HIV-1 infection and the antiviral activity of MX2.
Kane M, Rebensburg SV, Takata MA, Zang TM, Yamashita M, Kvaratskhelia M, Bieniasz PD. Kane M, et al. Elife. 2018 Aug 7;7:e35738. doi: 10.7554/eLife.35738. Elife. 2018. PMID: 30084827 Free PMC article. - Cyclophilin A potentiates TRIM5α inhibition of HIV-1 nuclear import without promoting TRIM5α binding to the viral capsid.
Burse M, Shi J, Aiken C. Burse M, et al. PLoS One. 2017 Aug 2;12(8):e0182298. doi: 10.1371/journal.pone.0182298. eCollection 2017. PLoS One. 2017. PMID: 28767697 Free PMC article.
References
- Ball J. R., Ullman K. S. 2005. Versatility at the nuclear pore complex: lessons learned from the nucleoporin Nup153. Chromosoma 114:319–330 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials