Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome - PubMed (original) (raw)
Multicenter Study
. 2011 Jul;60(7):1917-25.
doi: 10.2337/db10-1707. Epub 2011 May 18.
Susanne Famulla, Nina Wronkowitz, Sonja Hartwig, Stefan Lehr, D Margriet Ouwens, Kristin Eckardt, Jean M Kaufman, Mikael Ryden, Stefan Müller, Franz-Georg Hanisch, Johannes Ruige, Peter Arner, Henrike Sell, Juergen Eckel
Affiliations
- PMID: 21593202
- PMCID: PMC3121429
- DOI: 10.2337/db10-1707
Multicenter Study
Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome
Daniela Lamers et al. Diabetes. 2011 Jul.
Abstract
Objective: Comprehensive proteomic profiling of the human adipocyte secretome identified dipeptidyl peptidase 4 (DPP4) as a novel adipokine. This study assessed the functional implications of the adipokine DPP4 and its association to the metabolic syndrome.
Research design and methods: Human adipocytes and skeletal and smooth muscle cells were used to monitor DPP4 release and assess the effects of soluble DPP4 on insulin signaling. In lean and obese subjects, depot-specific expression of DPP4 and its release from adipose tissue explants were determined and correlated to parameters of the metabolic syndrome.
Results: Fully differentiated adipocytes exhibit a substantially higher release of DPP4 compared with preadipocytes or macrophages. Direct addition of DPP4 to fat and skeletal and smooth muscle cells impairs insulin signaling. A fivefold higher level of DPP4 protein expression was seen in visceral compared with subcutaneous fat of obese patients, with no regional difference in lean subjects. DPP4 serum concentrations significantly correlated with adipocyte size. By using adipose tissue explants from lean and obese subjects, we observed a twofold increase in DPP4 release that strongly correlated with adipocyte volume and parameters of the metabolic syndrome and was decreased to the lean level after weight reduction. DPP4 released from adipose tissue correlated positively with an increasing risk score for the metabolic syndrome.
Conclusions: DPP4 is a novel adipokine that may impair insulin sensitivity in an autocrine and paracrine fashion. Furthermore, DPP4 release strongly correlates with adipocyte size, potentially representing an important source of DPP4 in obesity. Therefore, we suggest that DPP4 may be involved in linking adipose tissue and the metabolic syndrome.
© 2011 by the American Diabetes Association.
Figures
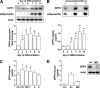
FIG. 1.
DPP4 protein level and release during adipocyte differentiation and after stimulation with different regulatory factors. A: Human primary adipocytes were differentiated as described in
research design and methods
, and DPP4 protein level during differentiation was analyzed by SDS-PAGE and Western blot. Adiponectin expression served as a control of differentiation. Data were normalized to the protein level of actin and are expressed relative to day 0. Data are mean values ± SEM, n ≥5, *P < 0.05 vs. preadipocytes. B: Detection of DPP4 at day 14 of differentiation using 1–5 μL of concentrated conditioned medium analyzed by SDS-PAGE and Western blot. Twenty-four–hour release of DPP4 by adipocytes determined at different time points of differentiation was analyzed by ELISA. Data are mean values ± SEM, n ≥5, *P < 0.05 vs. day 0. C: Differentiated adipocytes were treated with 5 μmol/L troglitazone, 10 ng TNF-α, 50 mmol/L insulin, 5 nmol/L adiponectin, or incubated under hypoxic conditions for 24 h. DPP4 release by differentiated adipocytes after indicated 24-h treatments as measured by ELISA. Data are mean values ± SEM, n ≥7, *P < 0.05 vs. control. D: DPP4 release by preadipocytes, differentiated adipocytes, and adipose tissue–derived and cultured human macrophages was analyzed by ELISA. Data are mean values ± SEM, n ≥3; 10 μg total lysates derived from adipocytes and macrophages were analyzed by SDS-PAGE and Western blot, and signals were detected by enhanced chemiluminescence. A, adiponectin; Ad, adipocyte; CM, conditioned medium; H, hypoxic; I, insulin; MØ, macrophage; Pre, preadipocyte; Tro, troglitazone.
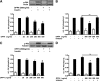
FIG. 2.
Effect of DPP4 on insulin-stimulated Akt phosphorylation in adipocytes and skeletal muscle cells. Differentiated human adipocytes (A and B) and skeletal muscle cells (C and D) were treated with the indicated amounts of DPP4 without and with concomitant administration of a specific DPP4 inhibitor for 24 h. After stimulation with insulin (100 nmol/L, 10 min), the cells were lysed and 5–10 μg of total lysates were resolved by SDS-PAGE and blotted to polyvinylidene fluoride membranes. Membranes were blocked with 5% milk in TBS containing 0.1% Tween 20 and incubated overnight with _p_-Akt antibody. After incubation with the appropriate HRP-coupled secondary antibody, the signal was detected by enhanced chemiluminescence. Signals were analyzed on a LUMI Imager Work Station (Boehringer). Data are actin normalized mean values ± SEM (n = 3–8). Representative Western blots are presented. For A, lanes were excised from a single Western blot and displayed in the presented order. Basal (white bars); insulin-stimulated (black bars). *Significantly different from insulin-stimulated control or indicated situation. ns, not significant.
FIG. 3.
Effect of DPP4 on insulin-stimulated Akt phosphorylation and proliferation in smooth muscle cells. A and B: Smooth muscle cells were treated with the indicated amounts of DPP4 without and with concomitant administration of a specific DPP4 inhibitor for 24 h. After stimulation with insulin (100 nmol/L, 10 min) the cells were lysed and Western blots performed as indicated in Fig. 2. Data are actin normalized mean values ± SEM (n = 3–6). Basal (white bars); insulin-stimulated (black bars). C: Proliferation of smooth muscle cells was determined by measuring the incorporation of BrdU into DNA. Data are expressed relative to the basal control value, taken as 100%. Data are mean values ± SEM (n = 3–8). ns, not significant. *Significantly different from control or indicated situation.
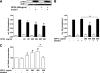
FIG. 4.
DPP4 serum concentration and expression in adipose tissue from lean compared with obese patients (clinical study 1). A: Sera from lean (n = 20) and morbidly obese (n = 20) men were analyzed for their DPP4 concentration by ELISA. Data are mean values ± SEM, *P < 0.05 vs. lean group. B: DPP4 protein level in adipose tissue biopsies was analyzed by SDS-PAGE and Western blot. Data were normalized to the protein level of actin and are expressed relative to subcutaneous adipose tissue from lean subjects. Data are mean values ± SEM, n = 8 for lean and n = 14 for obese patients, *P < 0.05 respective subcutaneous or designated group.
FIG. 5.
DPP4 serum concentrations correlate with various clinical and biochemical parameters (clinical study 1). Sera from lean (n = 20) and morbidly obese (n = 20) men were analyzed for their DPP4 concentration by ELISA. Linear regression analysis of DPP4 serum concentration and patient characteristics such as age (A), BMI (B), size of subcutaneous (C) and visceral (D) adipocytes, insulin concentration (E), adiponectin concentration (F), and leptin concentration (G). Statistical evaluation is indicated in each graph. vis, visceral.
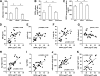
FIG. 6.
DPP4 release of explants obtained from adipose tissue of lean controls and obese patients before and after bariatric surgery, and linear correlation with various clinical and biochemical parameters (clinical study 2). A and B: Samples of adipose tissue were obtained from lean controls (n = 10) and obese patients before (n = 19) and after (n = 16) bariatric surgery, and used to generate explants as described in
research design and methods
. The size of adipocytes for each subject was measured (A). DPP4 release was analyzed by ELISA and related to the quantity of adipocytes (B). C: DPP4 serum concentration was measured in lean and obese patients before and after bariatric surgery. D–K: Linear regression analysis of DPP4 release per 107 cells and patient characteristics such as BMI (D), waist circumference (E), percent of body fat (F), HDL-cholesterol concentration (G), triglycerides concentration (H), HOMA (I), adipocyte volume (J), and leptin (K). A–C: Data are mean values ± SEM. *P < 0.05 between respective groups.
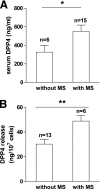
FIG. 7.
DPP4 in serum and release from adipose tissue explants in relation to a risk score for the metabolic syndrome. A risk score for the metabolic syndrome was calculated for all obese subjects in whom serum and adipose tissue explants were analyzed. Patients with a risk score of ≥3 were qualified as “with metabolic syndrome (MS).” Patients with a score of ≤2 were qualified as “without MS.” Data were analyzed using a t test. Data are mean values ± SEM. *P < 0.05, **P < 0.01.
Similar articles
- Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro.
Sell H, Blüher M, Klöting N, Schlich R, Willems M, Ruppe F, Knoefel WT, Dietrich A, Fielding BA, Arner P, Frayn KN, Eckel J. Sell H, et al. Diabetes Care. 2013 Dec;36(12):4083-90. doi: 10.2337/dc13-0496. Epub 2013 Oct 15. Diabetes Care. 2013. PMID: 24130353 Free PMC article. - Reduced DPP4 activity improves insulin signaling in primary human adipocytes.
Röhrborn D, Brückner J, Sell H, Eckel J. Röhrborn D, et al. Biochem Biophys Res Commun. 2016 Mar 11;471(3):348-54. doi: 10.1016/j.bbrc.2016.02.019. Epub 2016 Feb 10. Biochem Biophys Res Commun. 2016. PMID: 26872429 - DPP4 deletion in adipose tissue improves hepatic insulin sensitivity in diet-induced obesity.
Romacho T, Sell H, Indrakusuma I, Roehrborn D, Castañeda TR, Jelenik T, Markgraf D, Hartwig S, Weiss J, Al-Hasani H, Roden M, Eckel J. Romacho T, et al. Am J Physiol Endocrinol Metab. 2020 May 1;318(5):E590-E599. doi: 10.1152/ajpendo.00323.2019. Epub 2019 Dec 31. Am J Physiol Endocrinol Metab. 2020. PMID: 31891536 - Dipeptidyl Peptidase 4 (DPP4) as A Novel Adipokine: Role in Metabolism and Fat Homeostasis.
Barchetta I, Cimini FA, Dule S, Cavallo MG. Barchetta I, et al. Biomedicines. 2022 Sep 16;10(9):2306. doi: 10.3390/biomedicines10092306. Biomedicines. 2022. PMID: 36140405 Free PMC article. Review. - Multihormonal control of ob gene expression and leptin secretion from cultured human visceral adipose tissue: increased responsiveness to glucocorticoids in obesity.
Halleux CM, Servais I, Reul BA, Detry R, Brichard SM. Halleux CM, et al. J Clin Endocrinol Metab. 1998 Mar;83(3):902-10. doi: 10.1210/jcem.83.3.4644. J Clin Endocrinol Metab. 1998. PMID: 9506746 Review.
Cited by
- Adipo-myokines: two sides of the same coin--mediators of inflammation and mediators of exercise.
Raschke S, Eckel J. Raschke S, et al. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. Epub 2013 Jun 3. Mediators Inflamm. 2013. PMID: 23861558 Free PMC article. Review. - Comparison of the dipeptidyl peptidase-4 gene methylation levels between severely obese subjects with and without the metabolic syndrome.
Turcot V, Tchernof A, Deshaies Y, Pérusse L, Bélisle A, Marceau P, Hould FS, Lebel S, Vohl MC. Turcot V, et al. Diabetol Metab Syndr. 2013 Feb 4;5(1):4. doi: 10.1186/1758-5996-5-4. Diabetol Metab Syndr. 2013. PMID: 23379505 Free PMC article. - Obesidomics: contribution of adipose tissue secretome analysis to obesity research.
Pardo M, Roca-Rivada A, Seoane LM, Casanueva FF. Pardo M, et al. Endocrine. 2012 Jun;41(3):374-83. doi: 10.1007/s12020-012-9617-z. Epub 2012 Mar 21. Endocrine. 2012. PMID: 22434412 Review. - Vildagliptin vs liraglutide as a second-line therapy switched from sitagliptin-based regimens in patients with type 2 diabetes: A randomized, parallel-group study.
Takeshita Y, Takamura T, Kita Y, Otoda T, Kato K, Wakakuri H, Yamada M, Misu H, Matsushima Y, Kaneko S; Establishment of Rationale for Antiaging Diabetic Medicine (ERA-DM) Study Chapter 2 Group. Takeshita Y, et al. J Diabetes Investig. 2015 Mar;6(2):192-200. doi: 10.1111/jdi.12269. Epub 2014 Sep 9. J Diabetes Investig. 2015. PMID: 25802727 Free PMC article. - Extracellular Vesicles as Carriers of Adipokines and Their Role in Obesity.
Camino T, Lago-Baameiro N, Pardo M. Camino T, et al. Biomedicines. 2023 Feb 1;11(2):422. doi: 10.3390/biomedicines11020422. Biomedicines. 2023. PMID: 36830957 Free PMC article. Review.
References
- Sell H, Dietze-Schroeder D, Eckel J. The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol Metab 2006;17:416–422 - PubMed
- Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab 2003;14:137–145 - PubMed
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537–1545 - PubMed
- Breitling R. Robust signaling networks of the adipose secretome. Trends Endocrinol Metab 2009;20:1–7 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous