Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes - PubMed (original) (raw)
Comparative Study
Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes
Shiwei Wang et al. J Neurosci. 2011.
Abstract
Oligomannosidic glycans play important roles in nervous system development and function. By performing a phage display screening with oligomannose-specific antibodies, we identified an oligomannose-mimicking peptide that was functionally active in modulating neurite outgrowth and neuron-astrocyte adhesion. Using the oligomannose-mimicking peptide in crosslinking experiments, synapsin I was identified as a novel oligomannose-binding protein in mouse brain. Further analyses not only verified that synapsin I is an oligomannose-binding lectin, but also indicated that it is a glycoprotein carrying oligomannose and Lewis(x). We also found that synapsin I is expressed in glia-enriched cultures and is released from glial cells via exosomes. Incubation of glial-derived exosomes in the presence of high KCl concentrations or subjecting glial cell cultures to either oxygen/glucose deprivation or hydrogen peroxide resulted in release of synapsin I from exosomes. Application of synapsin I promoted neurite outgrowth from hippocampal neurons and increased survival of cortical neurons upon hydrogen peroxide treatment or oxygen/glucose deprivation. Coculture experiments using wild-type hippocampal neurons and wild-type or synapsin-deficient glial cells showed enhanced neurite outgrowth when synapsin was expressed by glial cells. Synapsin-induced neurite outgrowth was dependent on oligomannose on synapsin I and the neural cell adhesion molecule NCAM at the neuronal cell surface. The data indicate that, under conditions of high neuronal activity and/or oxidative stress, synapsin can be released from glial-derived exosomes and promotes neurite outgrowth and neuronal survival by modulating the interactions between glia and neurons.
Figures
Figure 1.
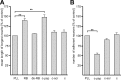
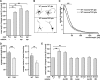
Characterization of a synthetic oligomannose-mimicking peptide. A, Binding of L3 and L4 antibodies to increasing amounts of substrate-coated catalase (c), catalase-coupled putative oligomannose-mimicking peptide (c-pep), or catalase-coupled scrambled peptide (c-scr) was determined by ELISA. B, L3 and L4 antibodies were preincubated with various amounts of untreated RNase B (RB) or EndoH-treated, deglycosylated RNase B (de-RB) and added to the substrate-coated putative oligomannose-mimicking peptide. Binding of the antibodies was determined by ELISA. Mean values ± SEM from at least three independent experiments are shown.
Figure 2.
The oligomannose-mimicking peptide promotes neurite outgrowth and disturbs adhesion of neurons to cortical astrocytes. A, Hippocampal neurons were grown on substrate-coated PLL alone or with catalase (c), catalase-coupled oligomannose-mimicking peptide (c-pep), catalase-coupled scrambled peptide (c-scr), RNase B (RB), or deglycosylated RNase B (de-RB). After 24 h in culture, cells were fixed and the length of the longest neurite per cell was determined. Mean length of the longest neurites obtained from PLL treatment was set to 100%. B, Freshly prepared GFP-expressing cerebellar granule neurons were preincubated with either solvent alone (control), catalase (c), catalase-coupled oligomannose-mimicking peptide (c-pep), or catalase-coupled scrambled peptide (c-scr) and subsequently added to a monolayer of cultured cortical astrocytes pretreated with solvent alone, catalase (c), catalase-coupled oligomannose-mimicking peptide (c-pep), or catalase-coupled scrambled peptide (c-scr). After removal of nonadherent cells, GFP-positive cells attached to the astrocyte monolayer were quantified. The mean number of adherent cells in the solvent-treated (PLL) control was set to 100%. Mean values ± SEM of three independent experiments are shown (**p < 0.01; one-way ANOVA followed by Dunnett's multiple-comparison test).
Figure 3.
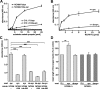
Synapsin is an oligomannose-specific lectin. Either catalase (c), catalase-coupled oligomannose-mimicking peptide (c-pep), or catalase-coupled scrambled peptide (c-scr) was conjugated to the biotin-carrying multifunctional and cleavable crosslinker sulfo-SBED. The conjugates were incubated with detergent extracts of crude brain homogenate. After UV crosslinking, proteins bound to the conjugates were isolated, separated by gel electrophoresis and subjected to Western blot analysis using HRP-coupled streptavidin (A), Coomassie Blue staining (CB) with the arrow indicating the position of the protein band identified as synapsin (B), or Western blot analysis with synapsin antibody (C). D, Synapsin preincubated in the absence (syn) or presence (syn+OM) of AMOG-derived oligomannose was incubated with substrate-coated untreated AMOG (AM) or EndoH-treated, deglycosylated AMOG (de-AM). The binding of synapsin was determined by ELISA using a synapsin antibody and background staining was subtracted. Mean values ± SEM of three independent experiments are indicated (**p < 0.01; one-way ANOVA followed by Dunnett's multiple-comparison test). E, Increasing amounts of AMOG (AM) or EndoH-treated, deglycosylated AMOG (de-AM) were incubated with substrate-coated synapsin. The binding of AMOG was determined by ELISA using an AMOG antibody and background staining was subtracted. Mean values ± SEM of three independent experiments are indicated.
Figure 4.
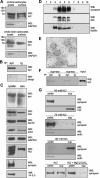
Synapsin is an oligomannose-bearing glycoprotein. Eluates from L3, L4, or control (ctrl) antibody columns were probed with oligomannose reactive L3 (A) or L4 (B) antibody or with synapsin antibody (C). The band around 50 kDa (arrow) represents the heavy chain of antibodies leaking from the column. D, Purified bovine synapsin was incubated in the absence (syn) or in the presence of EndoH (syn/H) or PNGase F (syn/F) and subjected to Western blot analysis using L3, L4, or synapsin antibody. E, F, Purified bovine synapsin was either subjected to Western blot analysis with antibodies against synapsin (syn) (E, F), HNK1 (HNK, 412) (E), polysialic acid (PSA) (E), and Lewisx (Lex) (E) or tested for lectin staining using Galanthus nivalis agglutinin (GNA), Datura stramonium agglutinin (DSA), peanut agglutinin (PNA), Sambucus nigra agglutinin (SNA), and Maackia amurensis agglutinin (MAA) (F).
Figure 5.
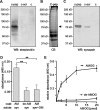
Synapsin is localized at the cell surface of cultured glial cells and present in glia-derived exosomes. A, Cultured cortical or whole-brain astrocytes were subjected to cell surface biotinylation and biotinylated cell surface proteins were isolated using streptavidin-conjugated beads. Lysates and biotinylated proteins (surface) were subjected to Western blot analysis (WB) with synapsin (syn) or GAPDH antibody. B, Biotinylated proteins were used for immunoprecipitation (IP) with synapsin antibody (syn) or nonimmune control antibody (Ig) and the resulting immunoprecipitates were subjected to Western blot analysis using HRP-coupled streptavidin (strept) or synapsin antibody (syn). C, Lysates and crude exosomal fractions (exo) isolated from the cell culture supernatant of cultured mouse cortical astrocytes by serial centrifugation were probed in Western blot analysis with synapsin, Alix, hsc70, actin, GAPDH, PDI, synaptophysin (synapto), or 14-3-3 antibodies. D, Crude exosomes were subjected to sucrose gradient centrifugation. After centrifugation fractions were collected and probed in Western blot analysis using synapsin, Alix, and flotillin antibodies. E, Fractions of exosomes that contain synapsin were analyzed by electron microscopy (scale bar, 100 nm). F, Cell-free cell culture supernatant of cortical astrocytes (sup+exo) or the supernatant obtained after 100,000 × g centrifugation of the cell culture supernatant of cortical astrocytes (sup-exo) were subjected to immunoprecipitation (IP) with synapsin antibody (syn) or nonimmune control antibody (Ig). The immunoprecipitates were subjected to Western blot (WB) analysis using synapsin (syn) antibody. G, Exosomes isolated from astrocyte culture supernatant were incubated in the presence of 60, 75, or 80 m
m
KCl without (KCl) or with 1.5 m
m
MgCl2 and CaCl2 (KCl+MgCl2/CaCl2). After incubation for 5 min at 37°C, samples were centrifuged at 100,000 × g to isolate exosomes (pellet) and proteins released from the exosomes (sup) by subjecting the supernatant to protein precipitation. The pellet and supernatant fractions were subjected to Western blot analysis using synapsin, actin, or PDI antibodies.
Figure 6.
Synapsin released from exosomes of cultured glia cells is neuroprotective under oxidative stress conditions. A, Cell culture supernatants from cortical and whole-brain glial cells cultured in the absence or presence of 20 μ
m
hydrogen peroxide (H2O2) were subjected to serial centrifugation. After centrifugation at 100,000 × g, the resulting pellets containing exosomes and supernatants (medium) containing soluble proteins were subjected to Western blot analysis with synapsin and Alix or GAPDH antibodies. The cells were also lysed and probed with synapsin and GAPDH antibodies in Western blot analysis. B, Cortical neurons were grown on substrate-coated PLL or synapsin in the absence or presence of 20 μ
m
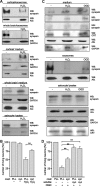
hydrogen peroxide (H2O2). After 24 h, neurons were stained with calcein and propidium iodide to determine the number of live and dead cells. Cell survival (ratio between number of live cells and total cell number) was calculated and survival of control cells was set to 100%. C, Cell culture supernatants from whole-brain glial cells subjected to glucose deprivation and either hypoxia (OGD) or hydrogen peroxide (H2O2) treatment were applied to serial centrifugation. After centrifugation at 100,000 × g, the resulting supernatants (medium) containing soluble proteins and pellets containing exosomes were subjected to Western blot analysis with synapsin, Alix, GAPDH, or calreticulin (CRT) antibodies. Cell lysates were probed with synapsin, Alix, calreticulin, and GAPDH antibodies. D, Cortical neurons grown on substrate-coated PLL or synapsin were subjected to oxygen/glucose deprivation (OGD) in the absence or presence of soluble synapsin. After 3 h, neurons were stained with calcein and propidium iodide to determine the number of live and dead cells. Cell survival was analyzed as described above (B). A, C, Representative blots out of at least two blots from at least three independent experiments are shown. C, Western blots of the cell culture supernatants probed with synapsin antibody from three independent experiments are shown. B, D, Mean values ± SEM of three independent experiments are shown (**p < 0.01 obtained by one-way ANOVA with Turkey's multiple-comparison test).
Figure 7.
Glia-derived synapsin promotes neurite outgrowth in an oligomannose-dependent manner. A, Hippocampal neurons were grown on substrate-coated PLL or synapsin (syn) in the absence (−) or presence of soluble synapsin. After fixation, the length of the longest neurite per cell was determined and value obtained with PLL coat in the absence of soluble synapsin was set to 100%. B–D, Wild-type (WT) hippocampal neurons were plated on mature wild-type or synapsin TKO glial cultures, maintained for 24 h, fixed, and stained with antibodies against neurofilament heavy chain and β-III tubulin to identify axons and the neurite network, respectively. B, Representative images of wild-type hippocampal neurons maintained on wild-type (top) or synapsin TKO glia (bottom). Scale bar, 10 μm. C, WT neurons grown on TKO glia show a simpler neurite network than neurons grown in parallel on WT glia. Sholl analysis was performed on n = 225 cells per genotype, from three independent experiments. The number of intersections (means ± SEM) is plotted as function of the distance from the cell body (***p < 0.001, Mann–Whitney U test). D, The average axonal length and the average number of axonal branching of wild-type (WT) hippocampal neurons maintained on either WT or synapsin TKO glia was determined and compared. Axons were identified as processes that were positive for neurofilament heavy chain immunostaining, and manually measured. n = 60 cells per genotype, from two independent experiments (**p < 0.01, ***p < 0.001, Mann–Whitney U test). E, Hippocampal neurons were grown on substrate-coated PLL alone (−), synapsin (syn), or EndoH-treated synapsin (de-syn) in the absence (−) or presence of untreated RNase B (RB), deglycosylated RNase B (de-RB), or catalase-coupled oligomannose-mimicking peptide (c-pep). The length of the longest neurite per cell was determined and the mean length of the longest neurites obtained with PLL coat was set to 100%. A, E, Mean values ± SEM of three independent experiments are shown (*p < 0.05; one-way ANOVA followed by Dunnett's multiple-comparison test).
Figure 8.
Synapsin binds to the extracellular domain of NCAM in an oligomannose-dependent manner and promotes neurite outgrowth in an NCAM-dependent manner. A, Increasing amounts of either untreated (syn) or EndoH-treated (de-syn) synapsin were substrate coated and incubated with either NCAM-Fc or CHL1-Fc. B, Substrate-coated untreated (syn) or EndoH-treated (de-syn) synapsin was incubated with increasing amounts of NCAM-Fc. C, Synapsin (syn) or synapsin treated with EndoH (de-syn) was substrate coated and incubated in the absence or presence of NCAM-Fc (NCAM), NCAM-Fc preincubated with AMOG (NCAM+AM), or NCAM preincubated with EndoH-deglycosylated AMOG (NCAM+de-AM). A–C, The binding of NCAM-Fc or CHL1 was determined by ELISA using a human Fc antibody. Mean values ± SEM of three independent experiments are shown (*p < 0.05, **p < 0.01, ***p < 0.001 one-way ANOVA followed by Dunnett's multiple-comparison test). D, Hippocampal neurons of NCAM-deficient (NCAM−/−) and wild-type (NCAM+/+) mice were grown on substrate-coated untreated (syn) or EndoH-treated (de-syn) synapsin. After 24 h in culture, cells were fixed and stained, and the length of the longest neurite per cell was determined. Mean length of the longest neurites obtained on PLL was set to 100%. Mean values ± SEM of two independent experiments are shown (**p < 0.01 one-way ANOVA followed by Dunnett's multiple-comparison test).
Figure 9.
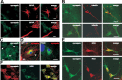
Synapsin is expressed by glial cells. A–E, Upon fixation and permeabilization, astrocyte-enriched glial cell cultures from whole brain of wild-type mice (A–C, E) or cortex of wild-type (D, F) or TKO (D) mice were subjected to double immunostaining with a mouse synapsin antibody and rabbit GFAP (A, D), β-III tubulin (B), or NG2 (E, F) antibodies. Secondary anti-mouse antibodies were labeled with the fluorescent dye Cy2 (A–C, E) or Cy3 (D, F), and secondary anti-rabbit antibodies were labeled with Cy3 (A–C, E) or Cy2 (D, F). Representative images of GFAP+/synapsin+ and GFAP−/synapsin+ glial cells (A), a tubulin+/synapsin+ neuron-like cell (B, top) and tubulin−/synapsin+ cells (B, bottom), synapsin+ neurons (C, arrow) and surrounding synapsin+ glial cells (C, arrowhead), GFAP+/synapsin+ glial cells (D, arrowhead), and synapsin+/NG2+ (E, F) and synapsin+/NG2− (F) glial cells are shown. D, Synapsin immunoreactivity (red) is shown in a GFAP+ wild-type (WT) astrocyte (arrowhead) as well as in a neuronal cell body (arrow) and along neurites (left), while no synapsin immunoreactivity is detectable in a GFAP+ TKO astrocytes (right). A–F, Scale bars, 50 μm.
Similar articles
- The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth.
Horstkorte R, Schachner M, Magyar JP, Vorherr T, Schmitz B. Horstkorte R, et al. J Cell Biol. 1993 Jun;121(6):1409-21. doi: 10.1083/jcb.121.6.1409. J Cell Biol. 1993. PMID: 8509458 Free PMC article. - Phosphorylation by PKA and Cdk5 Mediates the Early Effects of Synapsin III in Neuronal Morphological Maturation.
Piccini A, Perlini LE, Cancedda L, Benfenati F, Giovedì S. Piccini A, et al. J Neurosci. 2015 Sep 23;35(38):13148-59. doi: 10.1523/JNEUROSCI.1379-15.2015. J Neurosci. 2015. PMID: 26400944 Free PMC article. - Neuronal Exosomes Secreted under Oxygen-Glucose Deprivation/Reperfusion Presenting Differentially Expressed miRNAs and Affecting Neuronal Survival and Neurite Outgrowth.
Chiang CS, Fu SJ, Hsu CL, Jeng CJ, Tang CY, Huang YS, Tang SC. Chiang CS, et al. Neuromolecular Med. 2021 Sep;23(3):404-415. doi: 10.1007/s12017-020-08641-z. Epub 2021 Jan 3. Neuromolecular Med. 2021. PMID: 33389598 - Glia-derived exosomes: Promising therapeutic targets.
Li H, Luo Y, Zhu L, Hua W, Zhang Y, Zhang H, Zhang L, Li Z, Xing P, Zhang Y, Hong B, Yang P, Liu J. Li H, et al. Life Sci. 2019 Dec 15;239:116951. doi: 10.1016/j.lfs.2019.116951. Epub 2019 Oct 15. Life Sci. 2019. PMID: 31626787 Review. - The synapsins: multitask modulators of neuronal development.
Valtorta F, Pozzi D, Benfenati F, Fornasiero EF. Valtorta F, et al. Semin Cell Dev Biol. 2011 Jun;22(4):378-86. doi: 10.1016/j.semcdb.2011.07.008. Epub 2011 Jul 20. Semin Cell Dev Biol. 2011. PMID: 21798361 Review.
Cited by
- Emerging Roles of Extracellular Vesicles in the Central Nervous System: Physiology, Pathology, and Therapeutic Perspectives.
Gassama Y, Favereaux A. Gassama Y, et al. Front Cell Neurosci. 2021 Feb 23;15:626043. doi: 10.3389/fncel.2021.626043. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33708073 Free PMC article. Review. - Stress-Related Roles of Exosomes and Exosomal miRNAs in Common Neuropsychiatric Disorders.
Chamakioti M, Chrousos GP, Kassi E, Vlachakis D, Yapijakis C. Chamakioti M, et al. Int J Mol Sci. 2024 Jul 29;25(15):8256. doi: 10.3390/ijms25158256. Int J Mol Sci. 2024. PMID: 39125827 Free PMC article. Review. - Extracellular Vesicles Derived From Neural Stem Cells, Astrocytes, and Microglia as Therapeutics for Easing TBI-Induced Brain Dysfunction.
Hering C, Shetty AK. Hering C, et al. Stem Cells Transl Med. 2023 Mar 17;12(3):140-153. doi: 10.1093/stcltm/szad004. Stem Cells Transl Med. 2023. PMID: 36847078 Free PMC article. Review. - Exosome-mediated regulatory mechanisms in skeletal muscle: a narrative review.
Wang Z, Yang J, Sun X, Sun X, Yang G, Shi X. Wang Z, et al. J Zhejiang Univ Sci B. 2023 Jan 15;24(1):1-14. doi: 10.1631/jzus.B2200243. J Zhejiang Univ Sci B. 2023. PMID: 36632747 Free PMC article. Review.
References
- Bähler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed
- Bustos R, Kolen ER, Braiterman L, Baines AJ, Gorelick FS, Hubbard AL. Synapsin I is expressed in epithelial cells: localization to a unique trans-Golgi compartment. J Cell Sci. 2001;114:3695–3704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous