Synaptic glutamate release is modulated by the Na+ -driven Cl-/HCO₃⁻ exchanger Slc4a8 - PubMed (original) (raw)
Comparative Study
Synaptic glutamate release is modulated by the Na+ -driven Cl-/HCO₃⁻ exchanger Slc4a8
Anne Sinning et al. J Neurosci. 2011.
Abstract
On the one hand, neuronal activity can cause changes in pH; on the other hand, changes in pH can modulate neuronal activity. Consequently, the pH of the brain is regulated at various levels. Here we show that steady-state pH and acid extrusion were diminished in cultured hippocampal neurons of mice with a targeted disruption of the Na(+)-driven Cl(-)/HCO(3)(-) exchanger Slc4a8. Because Slc4a8 was found to predominantly localize to presynaptic nerve endings, we hypothesize that Slc4a8 is a key regulator of presynaptic pH. Supporting this hypothesis, spontaneous glutamate release in the CA1 pyramidal layer was reduced but could be rescued by increasing the intracellular pH. The reduced excitability in vitro correlated with an increased seizure threshold in vivo. Together with the altered kinetics of stimulated synaptic vesicle release, these data suggest that Slc4a8 modulates glutamate release in a pH-dependent manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1.
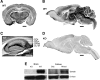
Neuronal expression of Slc4a8 in mouse brain. A, DAB staining of a coronal WT brain section with an antibody raised against Slc4a8. Scale bar, 1000 μm. B, Slc4a8 staining of a sagittal WT brain section. Scale bar, 1000 μm. C, All hippocampal layers stained positive for Slc4a8. Scale bar, 500 μm. SOr, Stratum oriens; SPyr, stratum pyramidale; SRad, stratum radiatum; SL-M, stratum lacunosum-moleculare. D, The specificity of the antibody was verified by the absence of signals in brain sections of KO mice. Scale bar, 1000 μm. E, The ∼120 kDa band for Slc4a8 was absent in KO brain lysate. Slc4a8 was detected in mixed hippocampal cultures from WT mice, but was not detectable in lysates from pure glia cell cultures.
Figure 2.
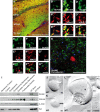
Presynaptic localization of Slc4a8 in the hippocampus. A, Costaining for Slc4a8 (green) and the presynaptic marker synaptophysin (Syn) (red) revealed a significant overlay. Scale bar, 200 μm. SOr, Stratum oriens; SPyr, stratum pyramidale; SRad, stratum radiatum; SL-M, stratum lacunosum-moleculare. B′–B‴, Higher magnification of SRad. Scale bar, 5 μm. C′–C‴, Slc4a8 (green) overlapped with the vesicular glutamate transporter vGLUT1 (red, SRad). Scale bar, 5 μm. D′–D‴, Similar results were obtained for vGLUT2 as shown in a close-up from the SRad. Scale bar, 5 μm. E′–E‴, Slc4a8 and the postsynaptic protein PSD-95 (red, SL-M) (scale bar, 5 μm) did not colocalize. F′–F‴, In agreement, costainings of Slc4a8 (green) and the dendritic marker MAP2 (red, SRad) (scale bar, 5 μm) did not support a dendritic expression. G′–G‴, Costaining of Slc4a8 (green) and GAD (red) revealed that Slc4a8 was not detected in most GABAergic nerve endings (SRad). Scale bar, 5 μm. H, Parvalbumin-positive interneurons (red) in the SPyr of the hippocampus did not express Slc4a8 (green). Scale bar, 50 μm. Nuclei were labeled with DAPI (blue). I, Immunoblot analysis of a density fractionation of WT mouse brain lysates. Slc4a8 was enriched in synaptosomes, synaptosomal membranes, and the synaptic junction plasma membrane fraction, but was not detectable in the postsynaptic density fraction. The postsynaptic protein PSD-95 and the presynaptic marker synaptophysin served as positive controls and β-actin as a loading control. J, Transmission electron microscopy of a freeze-fractured WT synaptosome immunogold labeled for Slc4a8 (large grains, 10 nm). K, The KO control confirmed the specificity of the Slc4a8 antibody. L, Colabeling of Slc4a8 (10 nm, white arrow) and the presynaptic marker syntaxin (small grains, 5 nm, black arrow) of freeze-fractured WT synaptosomes confirmed presynaptic localization of Slc4a8. Inset, Higher magnification. All images show the protoplasmic fracture face of a synaptosome membrane. Scale bars: J–L, 100 nm.
Figure 3.
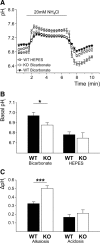
Impaired pH regulation in cultured hippocampal neurons of KO mice. A, Mean pHi of KO and WT neurons challenged with NH4Cl (WTHEPES, n = 9; WTBicarbonate, n = 35; KOBicarbonate, n = 35). B, Steady-state pHi was only reduced in KO neurons in the presence of bicarbonate (bicarbonate, p < 0.05; _n_ = 35/35; HEPES, _p_ > 0.05; n = 9/16). C, Peak alkalosis by NH4Cl was increased in KO neurons (ΔpHMaximum-Basal, p < 0.001; n = 35/35), but net acid load upon withdrawal of NH4Cl did not differ between genotypes (ΔpHBasal-Minimum, p = 0.97; n = 35/35). *p < 0.05; ***p < 0.0005.
Figure 4.
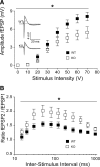
Reduction of network excitability in hippocampal slices from KO mice. A, CA1 population spike amplitudes upon stimulation of Schaffer collaterals were decreased in KO slices (p = 0.04; n = 22/19). Inset, Sample traces of somatic field recordings. Stimulus artifacts were omitted for clarity. B, Paired-pulse facilitation was significantly increased in slices of KO mice at 30–1000 ms interstimulus intervals (p = 0.03; n = 22/19). *p < 0.05.
Figure 5.
The frequency of mEPSCs but not of mIPSCs is decreased in KO mice in a pH-dependent manner. A, B, Representative traces of mEPSC recordings of WT and KO CA1 pyramidal cells at pHo 7.2. C, Cumulative plots of mEPSC amplitudes at varying pHi did not differ. Inset, Means of mEPSC amplitudes (p = 0.21; n = 32/15/38/14/7). D, Cumulative plots of interevent intervals revealed a shift to longer intervals in KO compared with WT (pHo 7.2, p < 0.001; n = 38/32). E, Shifting the pHo to 6.9 in WT diminished mEPSC frequencies (p < 0.0001; n = 32/15). F, Increasing pHi by decreasing pCO2 (pHo 7.5) or by application of the weak base TriMA raised the mEPSC frequency of KO cells (pHo 7.5, p = 0.03; n = 38/14; TriMA, p = 0.003; n = 38/7) toward WT. Inset, Means of mEPSC frequencies of WT and KO at different pHi. G, H, Representative traces of mIPSC recordings of WT and KO CA1 pyramidal neurons. I, J, Cumulative plots and means of mIPSC amplitude and frequencies did not differ between the genotypes (pHo 7.2, p = 0.78 and p = 0.27; n = 15/16). *p < 0.05.
Figure 6.
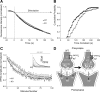
Impaired release of glutamate vesicles in KO mice. A, Time course of fluorescence destaining following field stimulation of cultured hippocampal WT and KO neurons upon vesicle labeling with FM1-43 (n = 54/50). B, Cumulative plot of the time constants revealed a shift toward a higher τ in KO neurons (p = 0.002; n = 54/50) equivalent with an increased mean time constant. C, Time course of the amplitude decrease of normalized NMDA currents in the presence of MK-801 (n = 8/6). Inset, Sample traces of NMDA receptor-mediated EPSCs recorded from CA1 pyramidal neurons in the presence of MK-801 upon repetitive stimulation of Schaffer collaterals. Mean time constant was increased in CA1 neurons of KO mice in the presence of MK-801. D, Model illustrating the consequences of Slc4a8 disruption on glutamate release. Slc4a8 is involved in presynaptic pH homeostasis. Upon disruption of Slc4a8, the intracellular pH is diminished and glutamate release impaired. Accordingly, fewer postsynaptic glutamate receptors are activated, but the pool of releasable vesicles appears to be unchanged.
Figure 7.
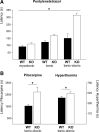
Slc4a8 deletion increases seizure threshold in different in vivo models of epilepsy. A, The latency until onset of pentylenetetrazol-induced myoclonic, tonic, and tonic-clonic seizures was significantly prolonged in KO mice (p < 0.0001; n = 22/22). B, Latency of ictal activity upon pilocarpine injection (p = 0.01; n = 13/13) was prolonged, as was seizure onset in a mouse pup model of hyperthermia-related epileptic activity (n = 11/10). *p < 0.05.
Similar articles
- Transporters involved in regulation of intracellular pH in primary cultured rat brain endothelial cells.
Taylor CJ, Nicola PA, Wang S, Barrand MA, Hladky SB. Taylor CJ, et al. J Physiol. 2006 Nov 1;576(Pt 3):769-85. doi: 10.1113/jphysiol.2006.117374. Epub 2006 Aug 17. J Physiol. 2006. PMID: 16916905 Free PMC article. - K+-Driven Cl-/HCO3- Exchange Mediated by Slc4a8 and Slc4a10.
Peña-Münzenmayer G, George AT, Llontop N, Mazola Y, Apablaza N, Spichiger C, Brauchi S, Sarmiento J, Zúñiga L, González W, Catalán MA. Peña-Münzenmayer G, et al. Int J Mol Sci. 2024 Apr 22;25(8):4575. doi: 10.3390/ijms25084575. Int J Mol Sci. 2024. PMID: 38674160 Free PMC article. - Lack of the sodium-driven chloride bicarbonate exchanger NCBE impairs visual function in the mouse retina.
Hilgen G, Huebner AK, Tanimoto N, Sothilingam V, Seide C, Garcia Garrido M, Schmidt KF, Seeliger MW, Löwel S, Weiler R, Hübner CA, Dedek K. Hilgen G, et al. PLoS One. 2012;7(10):e46155. doi: 10.1371/journal.pone.0046155. Epub 2012 Oct 9. PLoS One. 2012. PMID: 23056253 Free PMC article. - Regulation of Na+-independent Cl-/HCO3- exchangers by pH.
Alper SL, Chernova MN, Stewart AK. Alper SL, et al. JOP. 2001 Jul;2(4 Suppl):171-5. JOP. 2001. PMID: 11875255 Review. - The SLC4 family of HCO 3 - transporters.
Romero MF, Fulton CM, Boron WF. Romero MF, et al. Pflugers Arch. 2004 Feb;447(5):495-509. doi: 10.1007/s00424-003-1180-2. Epub 2004 Jan 14. Pflugers Arch. 2004. PMID: 14722772 Review.
Cited by
- Regulators of Slc4 bicarbonate transporter activity.
Thornell IM, Bevensee MO. Thornell IM, et al. Front Physiol. 2015 Jun 12;6:166. doi: 10.3389/fphys.2015.00166. eCollection 2015. Front Physiol. 2015. PMID: 26124722 Free PMC article. Review. - Disruption of Slc4a10 augments neuronal excitability and modulates synaptic short-term plasticity.
Sinning A, Liebmann L, Hübner CA. Sinning A, et al. Front Cell Neurosci. 2015 Jun 16;9:223. doi: 10.3389/fncel.2015.00223. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26136660 Free PMC article. - Disruption of KCC2 in Parvalbumin-Positive Interneurons Is Associated With a Decreased Seizure Threshold and a Progressive Loss of Parvalbumin-Positive Interneurons.
Herrmann T, Gerth M, Dittmann R, Pensold D, Ungelenk M, Liebmann L, Hübner CA. Herrmann T, et al. Front Mol Neurosci. 2022 Feb 3;14:807090. doi: 10.3389/fnmol.2021.807090. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35185464 Free PMC article. - Homeostatic plasticity and synaptic scaling in the adult mouse auditory cortex.
Teichert M, Liebmann L, Hübner CA, Bolz J. Teichert M, et al. Sci Rep. 2017 Dec 12;7(1):17423. doi: 10.1038/s41598-017-17711-5. Sci Rep. 2017. PMID: 29234064 Free PMC article. - Two central pattern generators from the crab, Cancer borealis, respond robustly and differentially to extreme extracellular pH.
Haley JA, Hampton D, Marder E. Haley JA, et al. Elife. 2018 Dec 28;7:e41877. doi: 10.7554/eLife.41877. Elife. 2018. PMID: 30592258 Free PMC article.
References
- Aram JA, Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-d-aspartate. Neurosci Lett. 1987;83:345–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous