Abrogation of donor T-cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL - PubMed (original) (raw)
. 2011 Jul 14;118(2):446-55.
doi: 10.1182/blood-2010-07-294785. Epub 2011 May 19.
Lucy W Kappel, Nury L Yim, Rebecca A Nejat, Gabrielle L Goldberg, Odette M Smith, Uttam K Rao, Lindsay Dykstra, Il-Kang Na, Amanda M Holland, Jarrod A Dudakov, Chen Liu, George F Murphy, Warren J Leonard, Glenn Heller, Marcel R M van den Brink
Affiliations
- PMID: 21596854
- PMCID: PMC3138694
- DOI: 10.1182/blood-2010-07-294785
Abrogation of donor T-cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL
Alan M Hanash et al. Blood. 2011.
Abstract
IL-21 is a proinflammatory cytokine produced by Th17 cells. Abrogation of IL-21 signaling has recently been shown to reduce GVHD while retaining graft-versus-leukemia/lymphoma (GVL) responses. However, the mechanisms by which IL-21 may lead to a separation of GVHD and GVL remain incompletely understood. In a murine MHC-mismatched BM transplantation model, we observed that IL-21 receptor knockout (IL-21R KO) donor T cells mediate decreased systemic and gastrointestinal GVHD in recipients of a transplant. This reduction in GVHD was associated with expansion of transplanted donor regulatory T cells and with tissue-specific modulation of Th-cell function. IL-21R KO and wild-type donor T cells showed equivalent alloactivation, but IL-21R KO T cells showed decreased infiltration and inflammatory cytokine production within the mesenteric lymph nodes. However, Th-cell cytokine production was maintained peripherally, and IL-21R KO T cells mediated equivalent immunity against A20 and P815 hematopoietic tumors. In summary, abrogation of IL-21 signaling in donor T cells leads to tissue-specific modulation of immunity, such that gastrointestinal GVHD is reduced, but peripheral T-cell function and GVL capacity are retained. IL-21 is thus an exciting target for therapeutic intervention and improvement of clinical transplantation outcomes.
Figures
Figure 1
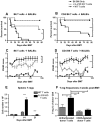
IL-21R is up-regulated on donor T cells early after BMT but is not required for efficient alloactivation. (A) WT B6 T cells were cultured in vitro for 24 hours in the presence or absence of PMA and ionomycin, then stained for FACS analysis of IL-21R levels. (B) WT B6 BM-TCD and T cells (1 × 106) were transplanted into lethally irradiated BALB/c recipients, which were killed on day 4 or day 7 after BMT. Splenocytes were harvested and stained for FACS analysis of donor T-cell IL-21R levels. Data are combined from 2 independent transplantations per time point, with 8-10 total recipients per group. **P < .01 and ***P < .001.
Figure 2
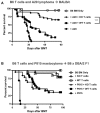
IL-21R KO T cells cause decreased GVHD and lead to increased regulatory T cells. (A) Lethally irradiated BALB/c mice were reconstituted with either BM-TCD only (n = 9), 1 × 106 IL-21R KO T cells + BM-TCD (n = 15), or 1 × 106 WT T cells + BM-TCD (n = 15). Curves represent combined data from 2 independent experiments. (B) Lethally irradiated BALB/c mice received either BM-TCD only (n = 9), 5 × 105 IL-21R KO CD4 T cells + BM-TCD (n = 15), or 5 × 105 WT CD4 T cells + BM-TCD (n = 15). Curves represent combined data from 2 independent experiments. (C,D) Transplant recipients were scored on a weekly basis for 5 clinical GVHD parameters: weight loss, activity, kyphosis, fur ruffling, and skin flaking. Scores range from 0 to 2 for each parameter, and animals are killed once a total score of 5 is attained. Curves represent the average score of each group at each time point. Shown are combined data from 2 independent experiments. (E) Lethally irradiated BALB/c mice were reconstituted with B6 BM-TCD and 1 × 106 B6 WT or 1 × 106 B6 IL-21R KO T cells. Spleens were harvested on days 7, 14, and 21 after BMT and stained for FACS analysis of donor CD4+CD25+Foxp3+ T-regs or CD4+Foxp3− nonregulatory T cells. Data represent 2 combined independent experiments on day 7 (n > 19), day 14 (n = 18), and day 21 (n = 15). (F) Lethally irradiated BALB/c mice were reconstituted with CD45.1 B6 BM-TCD and 5 × 105 CD45.2 B6 MACS-purified T cells. Transplanted T-cell populations were either unfractionated or depleted of T-regs by sorting for CD25− cells. Spleens were harvested on day 21 after BMT and analyzed by FACS for Foxp3 expression among donor CD4 T cells. Data represent 2 combined independent experiments with 8-11 total recipients per group. *P < .05, **P < .01, and ***P < .001 for WT versus KO T cells.
Figure 3
IL-21 signaling is not required for GVL against hematopoietic malignancy. (A) Lethally irradiated BALB/c mice were transplanted with either B6 BM-TCD alone or with A20 tumor cells (2.5-5 × 105) with or without WT versus IL-21R KO T cells (0.5-1 × 106). Recipients were followed for mortality, and curves represent combined data from 4 independent experiments (n = 19-20 for non–T-cell groups, n = 39 for T-cell groups). (B) Lethally irradiated B6 × DBA/2 F1 mice were transplanted with either B6 BM-TCD alone or with P815 tumor cells (1 × 103) with or without WT versus IL-21R KO T cells (1 × 106). Recipients were followed for mortality, and curves represent combined data from 2 independent experiments (n = 10 for non–T-cell groups, n = 20 for T-cell groups). ***P < .001 for WT versus KO T cells.
Figure 4
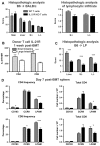
IL-21R KO T cells have decreased intestinal infiltration and LPAM expression after BMT. (A) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Skin, liver, small intestine (S.I.) and large intestine (L.I.) were harvested 3 weeks after BMT. H&E-stained slides were scored for histopathologic damage and lymphocyte infiltration. Shown is the mean of each group from 2 combined independent experiments (n > 12). (B) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT B6 T cells (1 × 106). Lamina propria lymphocytes from small and large intestines were isolated 1 week after BMT and stained for IL-21R expression on donor T cells. Shown are combined data from 2 independent experiments with 7 total recipients per group. (C) Lethally irradiated LP mice were transplanted with B6 BM-TCD and WT or IL-21R KO B6 T cells (1 × 106). Liver, small intestine, and large intestine were harvested 3 weeks after BMT, and H&E-stained slides were scored for histopathologic damage. Shown are combined data from 2 independent experiments with 17 total recipients per group. (D) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Splenocytes were harvested 1 week after BMT and stained for FACS analysis of donor T-cell CD103, CCR9, and LPAM. Shown are combined data from 4 to 5 independent experiments with 20-25 total recipients per group. *P < .05 and ***P < .001 for WT versus KO T cells (A,C,D) or WT T cells in small versus large intestine (B).
Figure 5
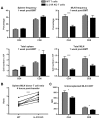
IL-21R KO T cells are decreased in MLNs after BMT. (A) Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). Spleens and MLNs were harvested 1 week after BMT and stained for FACS analysis of donor CD4 and CD8 T cells. Shown are combined data from 4 independent experiments with 20 total recipients per group. (B) CD45.1 B6 and IL-21R KO (CD45.2) T cells were cotransferred into lethally irradiated BALB/c recipients. Spleens and MLNs were harvested 4 hours after transfer and stained for FACS analysis of donor T cells. The ratio of spleen to MLN donor T cells was calculated, and connecting lines indicate WT (CD45.1) and KO (CD45.2) donor T cells isolated from individual recipients. Shown is 1 of 3 independent experiments. (C) Freshly isolated MLNs were harvested from untransplanted WT or CD45.1 B6 and IL-21R KO B6 mice and stained for FACS analysis of T-cell CCR7. Shown are combined results of 4 independent experiments with a total of 14 mice per group. *P < .05 and ***P < .001 for WT versus KO T cells.
Figure 6
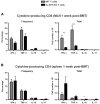
IL-21R KO CD4 T-cell IFN-γ and TNF-α are maintained in the spleen but reduced in MLNs. Lethally irradiated BALB/c mice were transplanted with B6 BM-TCD and WT or IL-21R KO T cells (1 × 106). (A) MLNs and (B) spleens were harvested 1 week after BMT and stained for intracellular cytokine FACS analysis of donor CD4 T cells. Shown are combined data from 4 independent experiments with 20 total recipients per group. **P < .01 and ***P < .001 for WT versus KO T cells.
Similar articles
- Targeting Interleukin-2-Inducible T-Cell Kinase (ITK) Differentiates GVL and GVHD in Allo-HSCT.
Mammadli M, Huang W, Harris R, Sultana A, Cheng Y, Tong W, Pu J, Gentile T, Dsouza S, Yang Q, Bah A, August A, Karimi M. Mammadli M, et al. Front Immunol. 2020 Nov 26;11:593863. doi: 10.3389/fimmu.2020.593863. eCollection 2020. Front Immunol. 2020. PMID: 33324410 Free PMC article. - IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation.
Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR, Ferrara JL. Teshima T, et al. J Clin Invest. 1999 Aug;104(3):317-25. doi: 10.1172/JCI7111. J Clin Invest. 1999. PMID: 10430613 Free PMC article. - Lack of IL-21 signal attenuates graft-versus-leukemia effect in the absence of CD8 T-cells.
Meguro A, Ozaki K, Hatanaka K, Oh I, Sudo K, Ohmori T, Matsu H, Tatara R, Sato K, Sakata Y, Nakae S, Leonard WJ, Ozawa K. Meguro A, et al. Bone Marrow Transplant. 2011 Dec;46(12):1557-65. doi: 10.1038/bmt.2010.342. Epub 2011 Jan 24. Bone Marrow Transplant. 2011. PMID: 21258423 Free PMC article. - Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.
Fowler DH, Gress RE. Fowler DH, et al. Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review. - The role of interleukin-12 in preserving the graft-versus-leukemia effect of allogeneic CD8 T cells independently of GVHD.
Yang YG, Sykes M. Yang YG, et al. Leuk Lymphoma. 1999 May;33(5-6):409-20. doi: 10.3109/10428199909058446. Leuk Lymphoma. 1999. PMID: 10342569 Review.
Cited by
- IFNγR signaling mediates alloreactive T-cell trafficking and GVHD.
Choi J, Ziga ED, Ritchey J, Collins L, Prior JL, Cooper ML, Piwnica-Worms D, DiPersio JF. Choi J, et al. Blood. 2012 Nov 8;120(19):4093-103. doi: 10.1182/blood-2012-01-403196. Epub 2012 Sep 12. Blood. 2012. PMID: 22972985 Free PMC article. - Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease.
Chang YJ, Zhao XY, Huang XJ. Chang YJ, et al. Front Immunol. 2018 Dec 21;9:3041. doi: 10.3389/fimmu.2018.03041. eCollection 2018. Front Immunol. 2018. PMID: 30619371 Free PMC article. Review. - Serum miR-29a Is Upregulated in Acute Graft-versus-Host Disease and Activates Dendritic Cells through TLR Binding.
Ranganathan P, Ngankeu A, Zitzer NC, Leoncini P, Yu X, Casadei L, Challagundla K, Reichenbach DK, Garman S, Ruppert AS, Volinia S, Hofstetter J, Efebera YA, Devine SM, Blazar BR, Fabbri M, Garzon R. Ranganathan P, et al. J Immunol. 2017 Mar 15;198(6):2500-2512. doi: 10.4049/jimmunol.1601778. Epub 2017 Feb 3. J Immunol. 2017. PMID: 28159900 Free PMC article. - Conformal Nanoencapsulation of Allogeneic T Cells Mitigates Graft-versus-Host Disease and Retains Graft-versus-Leukemia Activity.
Zhao S, Zhang L, Han J, Chu J, Wang H, Chen X, Wang Y, Tun N, Lu L, Bai XF, Yearsley M, Devine S, He X, Yu J. Zhao S, et al. ACS Nano. 2016 Jun 28;10(6):6189-200. doi: 10.1021/acsnano.6b02206. Epub 2016 May 31. ACS Nano. 2016. PMID: 27224853 Free PMC article. - Advances in the treatment of acute graft-versus-host disease.
Qian L, Wu Z, Shen J. Qian L, et al. J Cell Mol Med. 2013 Aug;17(8):966-75. doi: 10.1111/jcmm.12093. Epub 2013 Jun 26. J Cell Mol Med. 2013. PMID: 23802653 Free PMC article. Review.
References
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. - PubMed
- van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2(4):273–281. - PubMed
- Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. - PubMed
- Petrovic A, Alpdogan O, Willis LM, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103(4):1542–1547. - PubMed
- Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21(4):149–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL069929/HL/NHLBI NIH HHS/United States
- R01 CA107096/CA/NCI NIH HHS/United States
- P01-CA33049/CA/NCI NIH HHS/United States
- R01-HL095075/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01-HL069929/HL/NHLBI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01-CA107096/CA/NCI NIH HHS/United States
- R01-AI080455/AI/NIAID NIH HHS/United States
- R01 HL095075/HL/NHLBI NIH HHS/United States
- R01 AI080455/AI/NIAID NIH HHS/United States
- P01 CA033049/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials