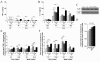Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study - PubMed (original) (raw)
Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study
Marie-Elsa Brochu et al. J Neuroinflammation. 2011.
Abstract
Background: Preterm and term newborns are at high risk of brain damage as well as subsequent cerebral palsy and learning disabilities. Indeed, hypoxia-ischemia (HI), pathogen exposures, and associated intracerebral increase of pro-inflammatory cytokines have all been linked to perinatal brain damage. However, the developmental effects of potential variations of pro- and anti-inflammatory cytokine ratios remain unknown.
Methods: Using rat models of perinatal brain damage induced by exposures to lipopolysaccharide (LPS) and/or HI at distinct levels of maturity, we compared cytokine expression at stages of cerebral development equivalent to either preterm (postnatal day 1, P1) or term (P12) newborns.
Results: At P1, expression of anti-inflammatory cytokine within the brain was either not modulated (IL-6, IL-10) or down-regulated (IL-1ra, TGF-β1) by HI, LPS or LPS+HI. In contrast, there was at P12 an up-regulation of all anti-inflammatory cytokines studied in HI or LPS+HI condition, but not after LPS exposure. Interestingly, IL-1β was the main pro-inflammatory cytokine up-regulated moderately at P1, and strongly at P12, with a weak co-expression of TNF-α observed mainly at P12. These age-dependant inflammatory reactions were also accompanied, under HI and LPS+HI conditions, at P12 only, by combined: (i) expression of chemokines CINC-1 and MCP-1, (ii) blood-brain barrier (BBB) leakage, and (iii) intracerebral recruitment of systemic immune cells such as neutrophils. In contrast, sole LPS induced IL-1β responses mainly within white matter at P1 and mainly within gray matter at P12, that were only associated with early MCP-1 (but no CINC-1) induction at both ages, without any recruitment of neutrophils and CD68+ cells.
Conclusion: HI and LPS+HI induce pro-inflammatory oriented immune responses in both preterm and term like brains, with a maximal inflammatory response triggered by the combination of LPS+HI. The profile of these neuroinflammatory responses presented striking variations according to age: no or down-regulated anti-inflammatory responses associated with mainly IL-1β release in preterm-like brains (P1), in sharp contrast to term-like brains (P12) presenting stronger anti-and pro-inflammatory responses, including both IL-1β and TNF-α releases, and BBB leakage. These developmental-dependant variations of neuroinflammatory response could contribute to the differential pattern of brain lesions observed across gestational ages in humans. This also highlights the necessity to take into consideration the maturation stage, of both brain and immune systems, in order to develop new anti-inflammatory neuroprotective strategies.
Figures
Figure 1
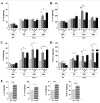
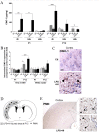
Comparison between P1 and P12 ELISA titrations of brain anti-inflammatory cytokines. Developmentally regulated induction of anti-inflammatory cytokines was shown after exposure to HI +/- LPS. An increased expression was detected after LPS and/or HI exposures at P12, but not at P1, for IL-10 (A), IL-1ra (B), IL-6 (C) and TGF-β1 (D). At P1, since the induction pattern was the same at 4, 24 or 48 h post-HI, only 48 h was shown. Control levels (E) of intracerebral cytokine titers were compared between P1 and P12. Protein detection was performed on 3 to 6 brains assayed in duplicate at each time point under each experimental condition. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Newman-Keuls post test (A-D) and t test with Welch correction (E).
Figure 2
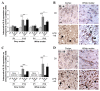
Comparison between P1 and P12 immunostaining intensities of IL-6 and IL-10 in brain. IL-6 and IL-10 expressions were increased only at P12 in brains of rat pups exposed to HI+/- LPS. Data are presented (A, C) as fold increase of IL-6 and IL-10 expressions compared to control (set at 1). (B) Increased IL-6 staining (arrowheads) in P12 spongiotic frontal cortex (pyknotic neurons with abnormal reduced contrast between nucleus and cytoplasm), and spongiotic underlying external capsule following LPS+HI exposure compared to control. (D) Increased IL-10 staining (arrowheads) in P12 lesioned frontal cortex and underlying external capsule exposed to LPS+HI compared to control. IHC was performed at 48 h post-HI, in 3 to 4 brains under each experimental condition. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Newman-Keuls post test. Scale bars = 15 μm (B, D)
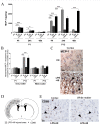
Figure 3
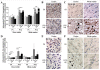
Comparison between P1 and P12 immunostaining intensities of TGF-β1 and IL-1ra in brain. TGF-β1 and IL-1ra expressions were decreased at P1 and conversely increased at P12 in brains of rat pups exposed to HI and/or LPS. Data are presented (A, D) as fold increase of TGF-β1 and IL-1ra expressions compared to control (set at 1). Decreased TGF-β1 (B) and IL-1ra (E) staining in P1 frontal cortex exposed to LPS plus HI versus control. Increased TGF-β1 (C) and IL-1ra (F) staining (arrowheads) in P12 lesioned frontal cortex (pyknotic neurons with abnormal reduced contrast between nucleus and cytoplasm), and underlying external capsule exposed to LPS+/-HI versus control. IHC was performed at 48 h post-HI, in 3 to 4 brains under each experimental condition. *p < 0.05, ***p < 0.001, one-way ANOVA with Newman-Keuls post test. Scale bars = 15 μm (B, C, E, F).
Figure 4
P1 versus P12 ELISA titrations and immunostaining intensities of cerebral pro-inflammatory cytokines, IL-1β and TNF-α. TNF-α was not detected by ELISA at P1 but an up-regulation - weaker than the one observed for IL-1β - was detected at P12 after HI and LPS+HI exposures by ELISA (A) and IHC in both white and gray matters (E). No modulation of combined pro- and active forms of IL-1β was detected by ELISA between the different experimental conditions at P1 (B), whereas the active form (17 kDa) of IL-1β as detected by western blot was increased in LPS+HI condition (C, D). Some foci of increased IL-1 immunostaining were detected by IHC in brain exposed to HI or LPS+HI, at 48 h post-HI (F). At P12, IHC results confirmed an increased IL-1β expression localized especially in gray, and in a lesser extent in white matter, of brains exposed to HI and LPS+HI (F) and a TNF-α increase was detected only in the LPS+HI brains (E) at P1. Protein detection was performed by ELISA on 3 to 4 brains assayed in duplicate, at each time point under each experimental condition. IHC and western blot were performed at 48 h post-HI, in 3 to 4 brains in each experimental condition. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Newman-Keuls post test, except for LPS versus control at P1 in gray matter using t test with Welch correction.
Figure 5
P1 versus P12 intracerebral expressions of CINC-1 and levels of neutrophil infiltrations. CINC-1 was detected by ELISA at 4 and 24 h after HI in P1 brains exposed to LPS+/-HI (A). No CINC-1 was detected at 48 h after HI in P1 brains exposed or unexposed to LPS+/-HI either by ELISA (A) or IHC (data not shown) at 48 h post-HI. In contrast, following HI or LPS+HI at P12, CINC-1 was up-regulated in brains at 4, 24 and 48 h post-HI as shown by ELISA (A) and IHC experiments (B, C); this increase of CINC-1 intracerebral expression (C, arrowheads) at P12 was present in the neocortical gray matter (C), but not in the underlying white matter. Neutrophils (E, arrowheads) were detected only at P12 - not at P1 -, at 48 h post LPS+HI, in the neocortex ipsilateral to ischemia, i.e. in the areas of neocortical damage as illustrated in a schema (D). Protein detection was performed by ELISA on 3 to 4 brains assayed in duplicate at each time point under each experimental condition. IHC was performed at 48 h post-HI, in 3 to 4 brains under each experimental condition. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Newman-Keuls post test, except for LPS+HI versus control at P12 (48 h) by ELISA using t test with Welch correction (A).Scale bars = 15 μm (C, E).
Figure 6
BBB leakage and brain damage. Distribution of increased albumin staining within right hemispheric gray and white matters exposed to HI, LPS or LPS+HI in P12 brains compared to control (A) correlated with injured areas (B, C). Brain injuries induced by HI and LPS+HI at P12 corresponded to infarcted areas (*) located in right carotidian territory; extent severity of infarct were maximum under LPS+HI compared to HI (B). Schematic presentation of distribution of BBB leakage at P12, including albumin extravasations, PMN and CD68+ cells infiltrations in HI and LPS+HI brains showing a topographic association between all these components of the neuroinflammatory response and brain damage (C). Brain damage at P1 was detected in both white and gray matter (cortex and basal ganglia) (D). In contrast to P12 lesion, P1 brains submitted to HI and LPS+HI presented laminar (D2, black arrowheads) or columnar (D3, black arrowheads) areas of neuronal necrosis (D2, D3, empty arrowheads), linear microhemorrhage (D3, arrows) and foci of white matter damage (D1, black arrowheads) combining spongiotic cavities (D1, empty arrowheads) and disorganization of cellular architecture (D1).
Figure 7
P1 versus P12 intracerebral expressions of MCP-1 and levels of CD68+ cells. MCP-1 was detected at 4 and 24 h post-HI in pups exposed to LPS +/-HI. No MCP-1 was detected 48 h after HI at P1 in brains exposed or unexposed to LPS+/-HI either by ELISA (A) or IHC (B) at 48 h post-HI. In contrast, following HI or LPS+HI at P12, MCP-1 was up-regulated in brains at 4, 24 and 48 h post-HI as shown by ELISA (A). IHC experiments (B, C) showed that this increased intracerebral MCP-1 expression (C, arrowheads) at P12 was only detected in the neocortex (C). CD68+ cells (E, arrowheads) were detected at P12 - not at P1 - at 48 h post LPS+HI in the neocortex and white matter ipsilateral to ischemia, i.e. in the areas of neocortical damage as illustrated in the schema (D). Protein detection was performed by ELISA on 3 to 4 brains assayed in duplicate at each time point under each experimental condition. IHC was performed at 48 h post-HI, in 3 to 4 brains under each experimental condition. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Newman-Keuls post test. Scale bars = 15 μm (C, E)
Similar articles
- Involvement of neuronal IL-1β in acquired brain lesions in a rat model of neonatal encephalopathy.
Savard A, Lavoie K, Brochu ME, Grbic D, Lepage M, Gris D, Sebire G. Savard A, et al. J Neuroinflammation. 2013 Sep 5;10:110. doi: 10.1186/1742-2094-10-110. J Neuroinflammation. 2013. PMID: 24007297 Free PMC article. - Neuronal self-injury mediated by IL-1β and MMP-9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia-ischemia.
Savard A, Brochu ME, Chevin M, Guiraut C, Grbic D, Sébire G. Savard A, et al. J Neuroinflammation. 2015 May 30;12:111. doi: 10.1186/s12974-015-0330-8. J Neuroinflammation. 2015. PMID: 26025257 Free PMC article. - Cerebral and hepatic inflammatory response after neonatal hypoxia-ischemia in newborn rats.
Bonestroo HJ, Nijboer CH, van Velthoven CT, Kavelaars A, Hack CE, van Bel F, Heijnen CJ. Bonestroo HJ, et al. Dev Neurosci. 2013;35(2-3):197-211. doi: 10.1159/000346685. Epub 2013 May 8. Dev Neurosci. 2013. PMID: 23689428 - Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults.
Hagberg H, Peebles D, Mallard C. Hagberg H, et al. Ment Retard Dev Disabil Res Rev. 2002;8(1):30-8. doi: 10.1002/mrdd.10007. Ment Retard Dev Disabil Res Rev. 2002. PMID: 11921384 Review. - Uncovering the Role of Inflammation with Asphyxia in the Newborn.
Dhillon SK, Gressens P, Barks J, Gunn AJ. Dhillon SK, et al. Clin Perinatol. 2024 Sep;51(3):551-564. doi: 10.1016/j.clp.2024.04.012. Epub 2024 May 22. Clin Perinatol. 2024. PMID: 39095095 Review.
Cited by
- Effects of hydrogen-rich saline in neuroinflammation and mitochondrial dysfunction in rat model of sepsis-associated encephalopathy.
Dumbuya JS, Li S, Liang L, Chen Y, Du J, Zeng Q. Dumbuya JS, et al. J Transl Med. 2022 Nov 26;20(1):546. doi: 10.1186/s12967-022-03746-4. J Transl Med. 2022. PMID: 36435787 Free PMC article. - Microbubble formulation influences inflammatory response to focused ultrasound exposure in the brain.
McMahon D, Lassus A, Gaud E, Jeannot V, Hynynen K. McMahon D, et al. Sci Rep. 2020 Dec 9;10(1):21534. doi: 10.1038/s41598-020-78657-9. Sci Rep. 2020. PMID: 33299094 Free PMC article. - A Window on the Study of Aversive Instrumental Learning: Strains, Performance, Neuroendocrine, and Immunologic Systems.
de Oliveira CC, Gouveia FV, de Castro MC, Kuroki MA, Dos Santos LC, Fonoff ET, Teixeira MJ, Otoch JP, Martinez RC. de Oliveira CC, et al. Front Behav Neurosci. 2016 Aug 24;10:162. doi: 10.3389/fnbeh.2016.00162. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27605910 Free PMC article. - Paediatric sepsis-associated encephalopathy (SAE): a comprehensive review.
Dumbuya JS, Li S, Liang L, Zeng Q. Dumbuya JS, et al. Mol Med. 2023 Feb 23;29(1):27. doi: 10.1186/s10020-023-00621-w. Mol Med. 2023. PMID: 36823611 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous