Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons - PubMed (original) (raw)
Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons
Chen Gu et al. J Biol Chem. 2011.
Abstract
Precise localization of axonal ion channels is crucial for proper electrical and chemical functions of axons. In myelinated axons, Kv1 (Shaker) voltage-gated potassium (Kv) channels are clustered in the juxtaparanodal regions flanking the node of Ranvier. The clustering can be disrupted by deletion of various proteins in mice, including contactin-associated protein-like 2 (Caspr2) and transient axonal glycoprotein-1 (TAG-1), a glycosylphosphatidylinositol-anchored cell adhesion molecule. However, the mechanism and function of Kv1 juxtaparanodal clustering remain unclear. Here, using a new myelin coculture of hippocampal neurons and oligodendrocytes, we report that tyrosine phosphorylation plays a critical role in TAG-1-mediated clustering of axonal Kv1.2 channels. In the coculture, myelin specifically ensheathed axons but not dendrites of hippocampal neurons and clustered endogenous axonal Kv1.2 into internodes. The trans-homophilic interaction of TAG-1 was sufficient to position Kv1.2 clusters on axonal membranes in a neuron/HEK293 coculture. Mutating a tyrosine residue (Tyr⁴⁵⁸) in the Kv1.2 C terminus or blocking tyrosine phosphorylation disrupted myelin- and TAG-1-mediated clustering of axonal Kv1.2. Furthermore, Kv1.2 voltage dependence and activation threshold were reduced by TAG-1 coexpression. This effect was eliminated by the Tyr⁴⁵⁸ mutation or by cholesterol depletion. Taken together, our studies suggest that myelin regulates both trafficking and activity of Kv1 channels along hippocampal axons through TAG-1.
Figures
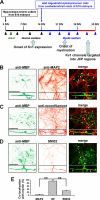
FIGURE 1.
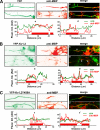
Myelin coculture of hippocampal neurons and oligodendrocytes. A, hippocampal neurons from day 18 rat embryos were dissociated and cultured for 14 days in regular neuron culture medium (indicated by black arrowheads). Dividing glial cells were eliminated by the Ara-C treatment (green arrowhead) for 2 days. Oligodendrocytes/precursor cells were dissociated from cerebellum and brain stem of day 18 rat embryos and added to the hippocampal neuron culture at 14 DIV. The neuron medium was replaced with the myelin medium (blue arrowheads), which was refreshed twice weekly. Myelination commenced after 1–2 weeks of coculture. Clear JXP targeting of endogenous Kv1.2 channels in myelinated hippocampal axons was observed after 2 weeks of coculture. B, myelin internodes (MBP-positive; green) did not colocalize with dendrites (MAP2-positive; red) of cultured hippocampal neurons at 28 DIV. C, myelin internodes (green) formed along axons (NF-positive; red). D, myelin (green) decreased the non-phosphorylated form of NFs (SMI32-positive; red) within the axonal segments underneath, consistent with the fact that myelin increases phosphorylated NFs in internodes. The cornered areas are shown in the lower panels. White arrows, MBP-positive myelin segments. Scale bars, 100 μm. E, summary of colocalization of MBP-positive myelin segments with MAP2 (4.8 ± 3.0%, n = 12), NF (93.8 ± 2.1%, n = 12), or non-phospho-NF (29.7 ± 4.2%, n = 13) staining. **, p < 0.001 (t test). Error bars, S.E.
FIGURE 2.
Myelin clusters endogenous Kv1.2 channels along axons of hippocampal neurons. A, myelin segments from myelinating oligodendrocytes clustered endogenous Kv1.2 channels along the axon of a mature hippocampal neuron in culture. Coculture of hippocampal neurons and oligodendrocytes was costained for endogenous Kv1.2 (green) and MBP (red) at 28 DIV. B, endogenous Kv1.2 was distributed smoothly along unmyelinated axons of a hippocampal neuron at 28 DIV. C, distribution patterns of Kv1.2 (green) and Nav (red) channels along unmyelinated axons of a hippocampal neuron at 28 DIV. In A–C, blue arrows, axons; blue arrowheads, dendrites; black arrows, myelin segments; black arrowhead, a myelin segment of an axon that did not express Kv1.2. D, immunofluorescence intensity profiles of Kv1.2 channels along the myelinated axon in A (red) and the unmyelinated axon in B (blue). E, immunofluorescence intensity profiles of Kv1.2 (blue) and Nav (red) channels along the unmyelinated axon in C. F, axonal Kv1.2 channels (green) were clustered in putative JXP regions in an MBP-positive myelin internode (red). White arrowheads, Kv1.2 clusters in putative JXP regions. G, Nav channels (green) localized in a heminode, indicated by a white arrowhead. H, segregation of Kv1 and Nav channels into the putative JXP and nodal regions, respectively. White arrowheads, putative nodes of Ranvier. I, summary of myelin internode length and percentage of the putative Kv1.2 JXP targeting at three developmental stages (n = 4 cocultures). Scale bars, 100 μm in A–C and 20 μm in F–H. **, p < 0.001 (t test). Error bars, S.E.
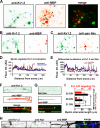
FIGURE 3.
The trans-homophilic interaction of TAG-1 directs the position of YFP-Kv1.2 clusters along axons. A, light microscopic image of coculture of hippocampal neurons and HEK293 cells. Transfected neurons (green arrows) at 8 DIV were cocultured with transfected HEK293 cells (blue arrow) for 1 day. B, the Kv1.2, Kvβ2, TAG-1, and Caspr2 constructs used in the neuron/HEK293 coculture. C, coculture of neurons transfected with YFP-Kv1.2 (green), Kvβ2, and TAG-1 (red) and HEK293 cells transfected with TAG-1 and mCherry (blue). The coculture was fixed, permeabilized, and stained with an anti-TAG-1 antibody (red in merge). D, YFP-Kv1.2 and TAG-1 are highly clustered in the region of contact between an axon expressing YFP-Kv1.2, Kvβ2, and TAG-1 and a HEK293 cell expressing TAG-1 and mCherry. E, TAG-1 from HEK293 cells failed to cluster YFP-Kv1.2 along the contacting axons expressing YFP-Kv1.2, Kvβ2, and Caspr2. White arrowheads, axonal segments contacting with transfected HEK293 cells. White arrows, coclusters of YFP-Kv1.2 and TAG-1 along axons not contacting with any HEK293 cells. Scale bars, 100 μm in A and C, 20 μm in D and E.
FIGURE 4.
Distinct targeting patterns of expressed TAG-1 and contactin in hippocampal neurons. TAG-1 (A and B) and contactin (C and D) were transfected into hippocampal neurons at 5 DIV. The neurons were costained with the dendritic marker MAP2 (A and C) or the axonal marker Tau1 (B and D) at 8 DIV. There was no clear signal for endogenous TAG-1 or contactin from untransfected neurons at this stage. E, fluorescence profiles of TAG-1 (A) and contactin (C) along axons (arrows). Scale bars, 50 μm.
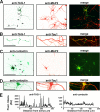
FIGURE 5.
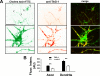
A putative tyrosine-based motif in the Kv1.2 C terminus is critical for clustering with TAG-1 on axonal membranes. A, diagram of constructs expressed in neurons. Hippocampal neurons cotransfected with Kv1.2HA constructs and GFP-TAG-1 were stained with anti-HA antibody under non-permeabilized conditions to reveal channel protein levels on axonal membranes. B, Kv1.2HA was highly colocalized with GFP-TAG-1 clusters on axonal membranes in the presence of expressed Kvβ2, as was Kv1.2HA alone (C) but not Kv1.2HAY458A (D). E, Kv1.2HAFCY alone was highly colocalized with GFP-TAG-1 clusters on axonal membranes. F, GFP-TAG-1 failed to induce Kv3.1bHA to form clusters along axonal membranes. G, Caspr2-mCh eliminated the large GFP-TAG-1 clusters, and both were distributed smoothly along axons. Fluorescence intensity profiles along the axons are shown on the right. White arrows, co-clusters. White arrowheads, clusters containing only one construct. H, co-expressed contactin (red) failed to cluster YFP-Kv1.2 (green) along axons. Scale bars, 100 μm in B, 20 μm in H.
FIGURE 6.
The tyrosine residue Tyr458 is critical for clustering Kv1.2 channels along hippocampal axons by myelin. Cultured hippocampal neurons were transfected at 5 DIV, cocultured with oligodendrocytes at 14 DIV, and fixed at 28 DIV. Myelin internodes were revealed by MBP staining (red). A, axons of hippocampal neurons transfected with YFP (green) were myelinated. The intensity of YFP fluorescence was not affected by myelin. B, YFP-Kv1.2 (green) was concentrated in internodes under myelin sheaths. C, myelin failed to cluster expressed YFP-Kv1.2Y458A (green) along axons. The fluorescence intensities of YFP and MBP staining along myelinated axonal segments are plotted in the lower panels. White arrows, myelin segments that contain clustered YFP-Kv1.2. The red bars indicate myelin internodes.
FIGURE 7.
Expressed TAG-1 increased the level of ganglioside GM1 on both axonal and dendritic membranes. A, hippocampal neurons were transfected with TAG-1 at 5 DIV, fixed, and stained with mouse monoclonal anti-TAG-1 (4D7) (red) and cholera toxin-FITC (green) under non-permeabilized conditions at 8 DIV. Two cornered areas in the upper panel are shown below. Scale bar, 100 μm. B, summary of the results. Shown is cholera toxin-FITC fluorescence on axonal membranes (control, 185.5 ± 25.8, n = 10; TAG-1, 360.2 ± 27.3, n = 10) and dendritic membranes (control, 350.6 ± 22.3, n = 10; TAG-1, 665.0 ± 66.2, n = 10). **, p < 0.01 (t test). Error bars, S.E.
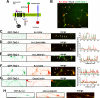
FIGURE 8.
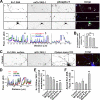
Tyrosine phosphorylation and related signaling events in TAG-1-mediated clustering of Kv1.2 along axons. Hippocampal neurons were cotransfected with Kv1.2HA, Kvβ2, and mCh-TAG-1 (red) at 5 DIV, fixed, and stained 3–5 days later. A, phosphotyrosine (phospho-Y) was highly enriched in the clusters containing both Kv1.2 and TAG-1. Transfected hippocampal neurons were stained with rabbit anti-Kv1.2 (green) and mouse anti-phosphotyrosine, 4G10 (blue), antibodies under permeabilized conditions. White arrows, clusters containing Kv1.2HA, mCh-TAG-1, and phosphotyrosine. The fluorescence intensity plots were given under high magnification images of three axonal segments. B, percentage of colocalization of phosphotyrosine (revealed by the 4G10 antibody), surface GM1 (revealed by the cholera toxin-FITC (CT-FITC) under non-permeabilized conditions), and F-actin (revealed by phalloidin Alexa 546), with clusters containing both Kv1.2 and TAG-1. Values are mean ± S.E. (error bars). **, p < 0.001 (t test). C, colocalization of surface GM1 with TAG-1/Kv1.2 clusters on axonal membranes. Transfected hippocampal neurons were stained with an anti-HA antibody (green) and cholera toxin-FITC (blue) under non-permeabilized conditions. White arrows, clusters containing Kv1.2HA, mCh-TAG-1, and CT-FITC. D, fluorescence profiles along the axon in C. E, differential effects of depleting cholesterol, depolymerizing F-actin, and inhibiting tyrosine kinases on TAG-1-induced clustering of Kv1.2HA on axonal membranes. The neurons were treated with 5 m
m
MβCD for 2 h or 4 days, 10 μ
m
cytochalasin B for 2 h, or 20 μ
m
genistein for 2 days or left untreated. F, anti-HA fluorescence intensities revealing the surface levels of Kv1.2HA on axonal membranes. *, p < 0.05; **, p < 0.01. One-way analysis of variance was used, followed by Dunnett's test for p values.
FIGURE 9.
TAG-1 coexpression regulates Kv1.2 channel activity. A, traces of whole-cell voltage clamp recording of the HEK293 cells transfected with Kv1.2 constructs. B, the G-V curve of Kv1.2HA was altered by coexpression of mCh-TAG-1 or cholesterol depletion (by the MβCD treatment) (top), and the effect of cholesterol depletion was eliminated by the Y458A mutation (bottom). C, current amplitudes and activation constants of Kv1.2 channel constructs under different conditions. **, p < 0.001 (t test). D, Kv1.2 channels on axonal membranes can be phosphorylated by tyrosine kinase(s) at Tyr458 in its C terminus, internalized into the axonal lumen, and then removed by transport and/or diffusion. E, glial TAG-1 from myelin membranes induces aggregation of axonal TAG-1, which leads to the formation of sphingolipid-cholesterol-rich clusters and F-actin patches, local activation of tyrosine kinase(s), and inhibition of phosphorylated Kv1.2 channels from being internalized. Error bars, S.E.
Similar articles
- Myelination of rodent hippocampal neurons in culture.
Gardner A, Jukkola P, Gu C. Gardner A, et al. Nat Protoc. 2012 Oct;7(10):1774-82. doi: 10.1038/nprot.2012.100. Epub 2012 Sep 6. Nat Protoc. 2012. PMID: 22955693 Free PMC article. - The Kv1-associated molecules TAG-1 and Caspr2 are selectively targeted to the axon initial segment in hippocampal neurons.
Pinatel D, Hivert B, Saint-Martin M, Noraz N, Savvaki M, Karagogeos D, Faivre-Sarrailh C. Pinatel D, et al. J Cell Sci. 2017 Jul 1;130(13):2209-2220. doi: 10.1242/jcs.202267. Epub 2017 May 22. J Cell Sci. 2017. PMID: 28533267 - Selective Axonal Expression of the Kv1 Channel Complex in Pre-myelinated GABAergic Hippocampal Neurons.
Bonetto G, Hivert B, Goutebroze L, Karagogeos D, Crépel V, Faivre-Sarrailh C. Bonetto G, et al. Front Cell Neurosci. 2019 May 16;13:222. doi: 10.3389/fncel.2019.00222. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31164806 Free PMC article. - Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease.
Pinatel D, Faivre-Sarrailh C. Pinatel D, et al. Life (Basel). 2020 Dec 24;11(1):8. doi: 10.3390/life11010008. Life (Basel). 2020. PMID: 33374190 Free PMC article. Review. - On the molecular architecture of myelinated fibers.
Arroyo EJ, Scherer SS. Arroyo EJ, et al. Histochem Cell Biol. 2000 Jan;113(1):1-18. doi: 10.1007/s004180050001. Histochem Cell Biol. 2000. PMID: 10664064 Review.
Cited by
- Myelination of rodent hippocampal neurons in culture.
Gardner A, Jukkola P, Gu C. Gardner A, et al. Nat Protoc. 2012 Oct;7(10):1774-82. doi: 10.1038/nprot.2012.100. Epub 2012 Sep 6. Nat Protoc. 2012. PMID: 22955693 Free PMC article. - Coupled left-shift of Nav channels: modeling the Na⁺-loading and dysfunctional excitability of damaged axons.
Boucher PA, Joós B, Morris CE. Boucher PA, et al. J Comput Neurosci. 2012 Oct;33(2):301-19. doi: 10.1007/s10827-012-0387-7. Epub 2012 Apr 5. J Comput Neurosci. 2012. PMID: 22476614 - Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation.
Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, Tabar V. Piao J, et al. Cell Stem Cell. 2015 Feb 5;16(2):198-210. doi: 10.1016/j.stem.2015.01.004. Cell Stem Cell. 2015. PMID: 25658373 Free PMC article. - Neuronal expression of GalNAc transferase is sufficient to prevent the age-related neurodegenerative phenotype of complex ganglioside-deficient mice.
Yao D, McGonigal R, Barrie JA, Cappell J, Cunningham ME, Meehan GR, Fewou SN, Edgar JM, Rowan E, Ohmi Y, Furukawa K, Furukawa K, Brophy PJ, Willison HJ. Yao D, et al. J Neurosci. 2014 Jan 15;34(3):880-91. doi: 10.1523/JNEUROSCI.3996-13.2014. J Neurosci. 2014. PMID: 24431446 Free PMC article. - Physiological and pathological functions of mechanosensitive ion channels.
Gu Y, Gu C. Gu Y, et al. Mol Neurobiol. 2014 Oct;50(2):339-47. doi: 10.1007/s12035-014-8654-4. Epub 2014 Feb 15. Mol Neurobiol. 2014. PMID: 24532247 Free PMC article. Review.
References
- Gu C., Jan Y. N., Jan L. Y. (2003) Science 301, 646–649 - PubMed
- Gu C., Zhou W., Puthenveedu M. A., Xu M., Jan Y. N., Jan L. Y. (2006) Neuron 52, 803–816 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous