Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system - PubMed (original) (raw)
. 2011 Jul;60(7):1882-93.
doi: 10.2337/db10-0427. Epub 2011 May 20.
Affiliations
- PMID: 21602515
- PMCID: PMC3121442
- DOI: 10.2337/db10-0427
Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system
Jean-Paul Kovalik et al. Diabetes. 2011 Jul.
Abstract
Objective: Adipocyte infiltration of the musculoskeletal system is well recognized as a hallmark of aging, obesity, and type 2 diabetes. Intermuscular adipocytes might serve as a benign storage site for surplus lipid or play a role in disrupting energy homeostasis as a result of dysregulated lipolysis or secretion of proinflammatory cytokines. This investigation sought to understand the net impact of local adipocytes on skeletal myocyte metabolism.
Research design and methods: Interactions between these two tissues were modeled using a coculture system composed of primary human adipocytes and human skeletal myotubes derived from lean or obese donors. Metabolic analysis of myocytes was performed after coculture with lipolytically silent or activated adipocytes and included transcript and metabolite profiling along with assessment of substrate selection and insulin action.
Results: Cocultured adipocytes increased myotube mRNA expression of genes involved in oxidative metabolism, regardless of the donor and degree of lipolytic activity. Adipocytes in the basal state sequestered free fatty acids, thereby forcing neighboring myotubes to rely more heavily on glucose fuel. Under this condition, insulin action was enhanced in myotubes from lean but not obese donors. In contrast, when exposed to lipolytically active adipocytes, cocultured myotubes shifted substrate use in favor of fatty acids, which was accompanied by intracellular accumulation of triacylglycerol and even-chain acylcarnitines, decreased glucose oxidation, and modest attenuation of insulin signaling.
Conclusions: The effects of cocultured adipocytes on myocyte substrate selection and insulin action depended on the metabolic state of the system. These findings are relevant to understanding the metabolic consequences of intermuscular adipogenesis.
© 2011 by the American Diabetes Association.
Figures
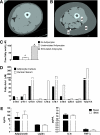
FIG. 1.
Adipocyte-myocyte coculture model. Computed tomography images of the thigh comparing (A) a young healthy female subject with (B) an older, obese female subject. a: Thigh muscle; b: subcutaneous adipose tissue; c: intermuscular adipose tissue. C: Free fatty acid concentration of the culture medium after 24-h exposure to basal (unstimulated) or stimulated (+100 μM IBMX) human adipocytes compared with no adipocytes. D: Individual free fatty acids in culture medium exposed to stimulated adipocytes for 24 h compared with no adipocytes. E: Adipokine concentrations in the culture medium after 24-h exposure to basal (unstimulated) or stimulated (+100 μM IBMX) human adipocytes. Results are expressed as mean ± SEM and representative of at least two experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test. FA, fatty acid. (A high-quality color representation of this figure is available in the online issue.)
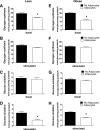
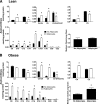
FIG. 2.
Adipocyte exposure alters glucose metabolism in primary human myotubes. Primary human skeletal myotubes from lean (A–D) and severely obese (E–H) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed, and myotubes were washed and incubated 2 h in α-MEM containing 2 μCi/mL
d
-[U-14C]glucose. Rates of glycogen synthesis and glucose oxidation were determined by measuring label incorporation into glycogen (A, B, E, and F) and CO2 (C, D, G, and H). Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
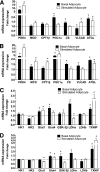
FIG. 3.
Effects of adipocyte exposure on fatty acid metabolism in primary human myotubes. Primary human skeletal myotubes from lean (A, B, and E) and severely obese (C, D, and F) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed, and myotubes were washed and incubated 2 h with α-MEM containing 100 μmol/L [14C]oleate (A–D) or 5 mmol/L [14C]glucose (E and F). Label incorporation into CO2 was measured to assess oxidation rates of fatty acid and glucose, respectively. The addition of 100 μmol/L etomoxir during the glucose oxidation assay (E and F) was used to block oxidation of intracellular lipids. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
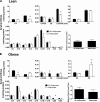
FIG. 4.
Adipocyte exposure alters acylcarnitine accumulation in primary human myotubes. Primary human skeletal myotubes from lean (A) and severely obese (B) women were maintained 24 h in the absence or presence of human adipocytes under basal conditions. After 24 h of coculture, adipocytes were removed, myotubes were washed, and cell lysates were prepared for acylcarnitine profiling by mass spectrometry. C3DC and C5OH are isobaric species. The ratio of total medium-chain relative to total long-chain acylcarnitines was calculated to assess potential flux limitations at the medium-chain acyl-CoA dehydrogenase step of β-oxidation. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. Abbreviations reflect carbon chain length (e.g., C2, acetylcarnitine). DC, dicarboxylic acid; OH, hydroxylated species. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
FIG. 5.
Effect of stimulated adipocyte exposure on acylcarnitine accumulation in primary human myotubes. Primary human skeletal myotubes from lean (A) and severely obese (B) women were maintained 24 h in the absence or presence of human adipocytes under stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed, myotubes were washed, and cell lysates were prepared for acylcarnitine profiling by mass spectrometry. C3DC and C5OH are isobaric species. The ratio of total medium-chain relative to total long-chain acylcarnitines was calculated to assess potential flux limitations at the medium-chain acyl-CoA dehydrogenase step of β-oxidation. Results are expressed as mean ± SEM and are representative of at least three experiments performed in triplicate. Abbreviations reflect carbon chain length (e.g., C2, acetylcarnitine). DC, dicarboxylic acid; OH, hydroxylated species. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
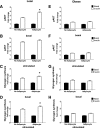
FIG. 6.
Adipocyte exposure alters myotube mRNA expression. Primary human skeletal myotubes from lean (A and C) and severely obese (B and D) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. After 24 h of coculture, adipocytes were removed and myotubes were washed and harvested for RNA extraction. Myotube expression of candidate mRNAs involved in fatty acid oxidation (A and B) or glucose metabolism (C and D) was measured by real-time quantitative PCR and normalized to mRNA levels of 18S. Data are expressed as mean ± SEM of the fold change in mRNA abundance relative to the control cells that were not exposed to adipocytes (dashed line). Results are representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test. CS, citrate synthase; Glut1, glucose transporter 1; Glut4, glucose transporter 4; HK1, hexokinase 1; HK2, hexokinase 2; LDHa, lactate dehydrogenase α; LDHb, lactate dehydrogenase β; MCD, malonyl-CoA decarboxylase; VLAD, very long-chain acyl-CoA dehydrogenase; TXNIP, thioredoxin-interacting protein.
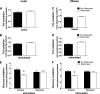
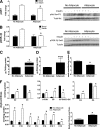
FIG. 7.
Coculture exposure for 24 h enhances myotube insulin response. Primary human skeletal myotubes from lean (A–D) and severely obese (E–H) women were maintained 24 h in the absence or presence of human adipocytes under basal or stimulated (100 μmol/L IBMX) conditions. Adipocytes were removed, and myotubes were washed and incubated with α-MEM ± 100 nmol/L insulin for 10 min. Protein extracts were used for immunoblot analysis of Akt Ser473 phosphorylation, corrected for total protein loading using Memcode staining. Measurement of total Akt protein showed no treatment effects. Rates of glycogen synthesis were measured after a 2-h incubation with α-MEM containing 5 mmol/L [14C]glucose ± 100 nmol/L insulin. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test.
FIG. 8.
Chronic coculture exposure dampens myotube insulin response. Primary human skeletal myotubes from lean women were maintained 96 h in the absence or presence of human adipocytes under stimulated (100 μmol/L IBMX) conditions. Adipocytes were removed, and myotubes were washed and incubated with α-MEM ± 100 nmol/L insulin for 10 min. A: Protein extracts were used for immunoblot analysis of Akt Ser473 phosphorylation, corrected for total protein loading using Memcode staining. Measurement of total Akt protein showed no treatment effects. B: GSK-3β Ser9 phosphorylation, corrected for total protein using tubulin staining. C–F: Myotube extracts were used for measurement of total triacylglycerol (C), total diacylglycerol (D), total ceramide (E), and acylcarnitine profiling (F). C3DC and C5OH are isobaric species. The ratio of total medium-chain relative to total long-chain acylcarnitines was calculated to assess potential flux limitations at the medium-chain acyl-CoA dehydrogenase step of β-oxidation. Results are expressed as mean ± SEM and representative of at least three experiments performed in triplicate, except for the TAG, DAG, and ceramides, which represent two independent experiments performed in quadruplicate. *Significant (P < 0.05) effect of adipocyte exposure analyzed by Student t test. Abbreviations reflect carbon chain length (e.g., C2, acetylcarnitine). DC, dicarboxylic acid; OH, hydroxylated species.
Similar articles
- Rosiglitazone ameliorates skeletal muscle insulin resistance by decreasing free fatty acids release from adipocytes.
Gong L, Jin H, Li Y, Quan Y, Yang J, Tang Q, Zou Z. Gong L, et al. Biochem Biophys Res Commun. 2020 Dec 17;533(4):1122-1128. doi: 10.1016/j.bbrc.2020.09.144. Epub 2020 Oct 6. Biochem Biophys Res Commun. 2020. PMID: 33036752 - Using a novel coculture model to dissect the role of intramuscular lipid load on skeletal muscle insulin responsiveness under reduced estrogen conditions.
Wohlers LM, Powers BL, Chin ER, Spangenburg EE. Wohlers LM, et al. Am J Physiol Endocrinol Metab. 2013 Jun 1;304(11):E1199-212. doi: 10.1152/ajpendo.00617.2012. Epub 2013 Apr 2. Am J Physiol Endocrinol Metab. 2013. PMID: 23548610 Free PMC article. - 1,2-Dilinoleoyl-sn-glycero-3-phosphocholine increases insulin sensitivity in palmitate-treated myotubes and induces lipolysis in adipocytes.
Park J, Jung TW, Chung YH, Park ES, Jeong JH. Park J, et al. Biochem Biophys Res Commun. 2020 Nov 26;533(1):162-167. doi: 10.1016/j.bbrc.2020.09.019. Epub 2020 Sep 14. Biochem Biophys Res Commun. 2020. PMID: 32943187 - Omega-3 fatty acids and adipose tissue biology.
Kuda O, Rossmeisl M, Kopecky J. Kuda O, et al. Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review.
Cited by
- Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes.
Miljkovic I, Cauley JA, Wang PY, Holton KF, Lee CG, Sheu Y, Barrett-Connor E, Hoffman AR, Lewis CB, Orwoll ES, Stefanick ML, Strotmeyer ES, Marshall LM; Osteoporotic Fractures in Men (MrOS) Research Group. Miljkovic I, et al. Obesity (Silver Spring). 2013 Oct;21(10):2118-25. doi: 10.1002/oby.20346. Epub 2013 May 29. Obesity (Silver Spring). 2013. PMID: 23408772 Free PMC article. - Translating the basic knowledge of mitochondrial functions to metabolic therapy: role of L-carnitine.
Marcovina SM, Sirtori C, Peracino A, Gheorghiade M, Borum P, Remuzzi G, Ardehali H. Marcovina SM, et al. Transl Res. 2013 Feb;161(2):73-84. doi: 10.1016/j.trsl.2012.10.006. Epub 2012 Nov 5. Transl Res. 2013. PMID: 23138103 Free PMC article. Review. - Comparison of Metabolic Network between Muscle and Intramuscular Adipose Tissues in Hanwoo Beef Cattle Using a Systems Biology Approach.
Lee HJ, Park HS, Kim W, Yoon D, Seo S. Lee HJ, et al. Int J Genomics. 2014;2014:679437. doi: 10.1155/2014/679437. Epub 2014 Nov 13. Int J Genomics. 2014. PMID: 25478565 Free PMC article. - Divergent effects of myogenic differentiation and diabetes on the capacity for muscle precursor cell adipogenic differentiation in a fibrin matrix.
Acosta FM, Jia UA, Stojkova K, Pacelli S, Brey EM, Rathbone C. Acosta FM, et al. Biochem Biophys Res Commun. 2020 May 21;526(1):21-28. doi: 10.1016/j.bbrc.2020.03.025. Epub 2020 Mar 17. Biochem Biophys Res Commun. 2020. PMID: 32192775 Free PMC article. - Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation.
Covington JD, Galgani JE, Moro C, LaGrange JM, Zhang Z, Rustan AC, Ravussin E, Bajpeyi S. Covington JD, et al. PLoS One. 2014 Mar 14;9(3):e91675. doi: 10.1371/journal.pone.0091675. eCollection 2014. PLoS One. 2014. PMID: 24632837 Free PMC article.
References
- Haslam DW, James WPT. Obesity. Lancet 2005;366:1197–1209 - PubMed
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005;111:1448–1454 - PubMed
- Després J.-P. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006;38:52–63 - PubMed
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981;30:1000–1007 - PubMed
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7–18 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R44-DK-07425302/DK/NIDDK NIH HHS/United States
- R01 AG028930/AG/NIA NIH HHS/United States
- R01 DK056112/DK/NIDDK NIH HHS/United States
- R01-AG-028930/AG/NIA NIH HHS/United States
- R01 DK073284/DK/NIDDK NIH HHS/United States
- R01-DK-56112/DK/NIDDK NIH HHS/United States
- R01-DK-073284/DK/NIDDK NIH HHS/United States
- R01 AG025205/AG/NIA NIH HHS/United States