Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement - PubMed (original) (raw)
- PMID: 21603045
- PMCID: PMC3090117
Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement
David Moher et al. Open Med. 2009.
No abstract available
Conflict of interest statement
Competing interests: None declared.
Figures
Box 1
Conceptual issues in the evolution from QUOROM to PRISMA
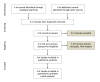
Figure 1
Flow of information through the different phases of a systematic review
Table 1
Checklist of items to include when reporting a systematic review or meta-analysis
Table 2
Substantive specific changes between the QUOROM checklist and the PRISMA checklist (a tick indicates the presence of the topic in QUOROM or PRISMA)
References
- Oxman AD, Cook DJ, Guyatt GH. Users' guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. JAMA. 1994;272:1367–1371. - PubMed
- Canadian Institutes of Health Research. Randomized controlled trials registration/application checklist (12/2006) 2006. [accessed 19 May 2009]. http://www.cihr-irsc.gc.ca/e/documents/rct_reg_e.pdf.
- Young C, Horton R. Putting clinical trials into context. Lancet. 2005;366:107. - PubMed
- Mulrow CD. The medical review article: State of the science. Ann Intern Med. 1987;106:485–488. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources