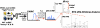Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells - PubMed (original) (raw)
Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells
Samuel A Myers et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The monosaccharide addition of an N-acetylglucosamine to serine and threonine residues of nuclear and cytosolic proteins (O-GlcNAc) is a posttranslational modification emerging as a general regulator of many cellular processes, including signal transduction, cell division, and transcription. The sole mouse O-GlcNAc transferase (OGT) is essential for embryonic development. To understand the role of OGT in mouse development better, we mapped sites of O-GlcNAcylation of nuclear proteins in mouse embryonic stem cells (ESCs). Here, we unambiguously identify over 60 nuclear proteins as O-GlcNAcylated, several of which are crucial for mouse ESC cell maintenance. Furthermore, we extend the connection between OGT and Polycomb group genes from flies to mammals, showing Polycomb repressive complex 2 is necessary to maintain normal levels of OGT and for the correct cellular distribution of O-GlcNAc. Together, these results provide insight into how OGT may regulate transcription in early development, possibly by modifying proteins important to maintain the ESC transcriptional repertoire.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
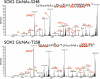
ETD MS/MS spectra of the two positional isomers of the _O_-GlcNAcylated SOX2 peptide. Adequate c- and z-series ions allow the unambiguous site assignment of the HexNAc mass modification to serine 248 (Upper) and threonine 258 (Lower). Charged reduced species and common neutral losses (55) are not labeled.
Fig. 2.
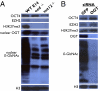
Western blot analysis of the relationship between PRC2 and OGT. (A) Disruption of PRC2 by mutations in either two, eed or suz12, of the three core components abrogates complex formation and methyltransferase activity as seen by EZH2 levels (third core component) and H3K27me3 levels, respectively. However, the pluripotency marker, OCT4, is unaffected. OGT and general _O_-GlcNAc levels are altered by PRC2 disruption. (B) siRNA knockdown of OGT does not significantly affect levels of OCT4, EZH2, or H3K27me3.
Fig. 3.
SILAC LWAC liquid chromatography-MS/MS workflow. Nonquantitative samples were prepared similarly, except a single cell type was used. LC, liquid chromatography; LTQ, linear trap quadrupole.
Fig. 4.
Diagram for landmarks of HCF-1. A zoomed-in view of the basic region and region of alternative proteolysis shows the extent and location of _O_-GlcNAc sites identified in this study. Numbers indicate landmark residues (Lower) or _O_-GlcNAc sites (Upper). g, glycosylation; NLS, nuclear localization sequence. Adapted from Wysocka et al. (44).
Similar articles
- Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets.
Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG 2nd, Shabanowitz J, Stanley P, Hart GW, Hunt DF, Yang F, Smith RD. Alfaro JF, et al. Proc Natl Acad Sci U S A. 2012 May 8;109(19):7280-5. doi: 10.1073/pnas.1200425109. Epub 2012 Apr 19. Proc Natl Acad Sci U S A. 2012. PMID: 22517741 Free PMC article. - Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis.
Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Wang Z, et al. Sci Signal. 2010 Jan 12;3(104):ra2. doi: 10.1126/scisignal.2000526. Sci Signal. 2010. PMID: 20068230 Free PMC article. - Effects of hypo-_O_-GlcNAcylation on Drosophila development.
Mariappa D, Ferenbach AT, van Aalten DMF. Mariappa D, et al. J Biol Chem. 2018 May 11;293(19):7209-7221. doi: 10.1074/jbc.RA118.002580. Epub 2018 Mar 27. J Biol Chem. 2018. PMID: 29588363 Free PMC article. - A critical perspective of the diverse roles of O-GlcNAc transferase in chromatin.
Gambetta MC, Müller J. Gambetta MC, et al. Chromosoma. 2015 Dec;124(4):429-42. doi: 10.1007/s00412-015-0513-1. Epub 2015 Apr 18. Chromosoma. 2015. PMID: 25894967 Free PMC article. Review. - Antibodies and activity measurements for the detection of O-GlcNAc transferase and assay of its substrate, UDP-GlcNAc.
Lefebvre T, Drougat L, Olivier-Van Stichelen S, Michalski JC, Vercoutter-Edouart AS. Lefebvre T, et al. Methods Mol Biol. 2013;1022:147-59. doi: 10.1007/978-1-62703-465-4_12. Methods Mol Biol. 2013. PMID: 23765660 Review.
Cited by
- Nutrient-sensitive protein O-GlcNAcylation shapes daily biological rhythms.
Liu X, Chiu JC. Liu X, et al. Open Biol. 2022 Sep;12(9):220215. doi: 10.1098/rsob.220215. Epub 2022 Sep 14. Open Biol. 2022. PMID: 36099933 Free PMC article. Review. - O-GlcNAc cycling: a link between metabolism and chronic disease.
Bond MR, Hanover JA. Bond MR, et al. Annu Rev Nutr. 2013;33:205-29. doi: 10.1146/annurev-nutr-071812-161240. Epub 2013 Apr 29. Annu Rev Nutr. 2013. PMID: 23642195 Free PMC article. Review. - Interplay between Metabolites and the Epigenome in Regulating Embryonic and Adult Stem Cell Potency and Maintenance.
Harvey A, Caretti G, Moresi V, Renzini A, Adamo S. Harvey A, et al. Stem Cell Reports. 2019 Oct 8;13(4):573-589. doi: 10.1016/j.stemcr.2019.09.003. Stem Cell Reports. 2019. PMID: 31597110 Free PMC article. Review. - Reversible developmental stasis in response to nutrient availability in the Xenopus laevis central nervous system.
McKeown CR, Thompson CK, Cline HT. McKeown CR, et al. J Exp Biol. 2017 Feb 1;220(Pt 3):358-368. doi: 10.1242/jeb.151043. Epub 2016 Nov 10. J Exp Biol. 2017. PMID: 27875263 Free PMC article. - Deciphering the Properties and Functions of Glycoproteins Using Quantitative Proteomics.
Xu S, Xu X, Wu R. Xu S, et al. J Proteome Res. 2023 Jun 2;22(6):1571-1588. doi: 10.1021/acs.jproteome.3c00015. Epub 2023 Apr 3. J Proteome Res. 2023. PMID: 37010087 Free PMC article. Review.
References
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence forO-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. - PubMed
- Yang X, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. - PubMed
- Tallent MK, et al. In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J Biol Chem. 2009;284:174–181. - PubMed
- Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010;584:2526–2538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous