MicroRNAs in addiction: adaptation's middlemen? - PubMed (original) (raw)
Review
. 2011 Dec;16(12):1159-68.
doi: 10.1038/mp.2011.58. Epub 2011 May 24.
Affiliations
- PMID: 21606928
- PMCID: PMC4251867
- DOI: 10.1038/mp.2011.58
Review
MicroRNAs in addiction: adaptation's middlemen?
M D Li et al. Mol Psychiatry. 2011 Dec.
Abstract
A central question in addiction is how drug-induced changes in synaptic signaling are converted into long-term neuroadaptations. Emerging evidence reveals that microRNAs (miRNAs) have a distinct role in this process through rapid response to cellular signals and dynamic regulation of local mRNA transcripts. Because each miRNA can target hundreds of mRNAs, relative changes in the expression of miRNAs can greatly impact cellular responsiveness, synaptic plasticity and transcriptional events. These diverse consequences of miRNA action occur through coordination with genes implicated in addictions, the most compelling of these being the neurotrophin BDNF, the transcription factor cAMP-responsive element-binding protein (CREB) and the DNA-binding methyl CpG binding protein 2 (MeCP2). In this study, we review the recent progress in the understanding of miRNAs in general mechanisms of plasticity and neuroadaptation and then focus on specific examples of miRNA regulation in the context of addiction. We conclude that miRNA-mediated gene regulation is a conserved means of converting environmental signals into neuronal response, which holds significant implications for addiction and other psychiatric illnesses.
Figures
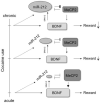
Figure 1
Relation between miR-212, methyl CpG-binding protein (MeCP2), and brain-derived neurotrophic factor (BDNF) mediates the adaptive response to chronic cocaine exposure. Cocaine increases BDNF concentrations, even after a single dose, and BDNF has a strong role in the motivating and rewarding aspects of the drug. BDNF signaling at the synapse increases transcription of miR-212 via an extracellular-signal-related kinase (ERK1/2) pathway. This miRNA exhibits mutual inhibition with MeCP2, a transcription factor necessary for BDNF expression in response to neural activity. The increased expression of miR-212 observed after chronic cocaine treatment therefore represents a mechanism of tolerance by inhibiting activity-dependent BDNF transcription in the nucleus accumbens.
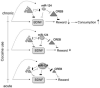
Figure 2
Decreased miR-124 expression in chronic cocaine conditions allows sustained increases in cAMP response element-binding protein (CREB). miR-124 is important in learning and memory through its inhibition of CREB. Following chronic cocaine exposure, this miRNA is down-regulated, whereas two of its targets, CREB and RE1-silencing transcription factor (REST), are upregulated. Both miR-124 and REST inhibit BDNF expression, so it seems that downregulation of this miRNA marks a shift in the control of BDNF inhibition from miR-124 to REST. This shift allows higher concentrations of CREB, which decreases the rewarding effects of cocaine.
Similar articles
- MicroRNAs regulate synaptic plasticity underlying drug addiction.
Smith ACW, Kenny PJ. Smith ACW, et al. Genes Brain Behav. 2018 Mar;17(3):e12424. doi: 10.1111/gbb.12424. Epub 2017 Oct 10. Genes Brain Behav. 2018. PMID: 28873276 Free PMC article. Review. - microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity.
Chandrasekar V, Dreyer JL. Chandrasekar V, et al. Mol Cell Neurosci. 2009 Dec;42(4):350-62. doi: 10.1016/j.mcn.2009.08.009. Epub 2009 Aug 22. Mol Cell Neurosci. 2009. PMID: 19703567 - Molecular and genetic substrates linking stress and addiction.
Briand LA, Blendy JA. Briand LA, et al. Brain Res. 2010 Feb 16;1314:219-34. doi: 10.1016/j.brainres.2009.11.002. Epub 2009 Nov 10. Brain Res. 2010. PMID: 19900417 Free PMC article. Review. - MicroRNAs and synaptic plasticity--a mutual relationship.
Aksoy-Aksel A, Zampa F, Schratt G. Aksoy-Aksel A, et al. Philos Trans R Soc Lond B Biol Sci. 2014 Sep 26;369(1652):20130515. doi: 10.1098/rstb.2013.0515. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25135976 Free PMC article. Review. - MicroRNAs and Synaptic Plasticity: From Their Molecular Roles to Response to Therapy.
Mohammadi AH, Seyedmoalemi S, Moghanlou M, Akhlagh SA, Talaei Zavareh SA, Hamblin MR, Jafari A, Mirzaei H. Mohammadi AH, et al. Mol Neurobiol. 2022 Aug;59(8):5084-5102. doi: 10.1007/s12035-022-02907-2. Epub 2022 Jun 6. Mol Neurobiol. 2022. PMID: 35666404 Review.
Cited by
- Global Approaches to the Role of miRNAs in Drug-Induced Changes in Gene Expression.
Eipper-Mains JE, Eipper BA, Mains RE. Eipper-Mains JE, et al. Front Genet. 2012 Jun 13;3:109. doi: 10.3389/fgene.2012.00109. eCollection 2012. Front Genet. 2012. PMID: 22707957 Free PMC article. - Epigenetics, microRNA, and addiction.
Kenny PJ. Kenny PJ. Dialogues Clin Neurosci. 2014 Sep;16(3):335-44. doi: 10.31887/DCNS.2014.16.3/pkenny. Dialogues Clin Neurosci. 2014. PMID: 25364284 Free PMC article. Review. - Non-Coding RNAs Regulating Morphine Function: With Emphasis on the In vivo and In vitro Functions of miR-190.
Zheng H, Law PY, Loh HH. Zheng H, et al. Front Genet. 2012 Jun 15;3:113. doi: 10.3389/fgene.2012.00113. eCollection 2012. Front Genet. 2012. PMID: 22715342 Free PMC article. - Epigenetics and psychostimulant addiction.
Schmidt HD, McGinty JF, West AE, Sadri-Vakili G. Schmidt HD, et al. Cold Spring Harb Perspect Med. 2013 Mar 1;3(3):a012047. doi: 10.1101/cshperspect.a012047. Cold Spring Harb Perspect Med. 2013. PMID: 23359110 Free PMC article. Review. - Morphine and microRNA Activity: Is There a Relation with Addiction?
Rodríguez RE. Rodríguez RE. Front Genet. 2012 Nov 9;3:223. doi: 10.3389/fgene.2012.00223. eCollection 2012. Front Genet. 2012. PMID: 23162566 Free PMC article.
References
- Perkins DO, Jeffries C, Sullivan P. Expanding the ‘central dogma’: the regulatory role of nonprotein coding genes and implications for the genetic liability to schizophrenia. Mol Psychiatry. 2005;10:69–78. - PubMed
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. - PubMed
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. - PubMed