mTOR-STAT3-notch signalling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism - PubMed (original) (raw)
mTOR-STAT3-notch signalling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism
Wei Ge et al. J Cell Mol Med. 2012 Mar.
Abstract
Mitochondrial aldehyde dehydrogenase-2 (ALDH2) has been shown to benefit myopathic changes following alcohol intake, although the precise mechanism is still unclear. This study was designed to evaluate the role of ALDH2 on chronic alcohol intake-induced myocardial geometric and functional damage with a focus on autophagic signalling. Wild-type friendly virus B (FVB) and transgenic mice overexpressing ALDH2 driven by chicken β-actin promoter were fed a 4% alcohol liquid diet for 12 weeks. Cardiac geometry and function were assessed using echocardiographic and IonOptix systems. Western blot analysis was used to evaluate the essential autophagy markers, Akt and AMP-dependent protein kinase (AMPK) as well as their downstream signalling mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3). Alcohol intake altered cardiac geometry and function as demonstrated by lessened LV wall and septal thickness, enlarged end systolic and diastolic diameters, decreased fractional shortening and cell shortening, the effects of which were mitigated by ALDH2 transgene. Chronic alcohol intake triggered myocardial autophagy as shown by LC3B II isoform switch, as well as decreased phosphorylation of mTOR, the effects of which were ablated by ALDH2. Chronic alcohol intake suppressed phosphorylation of Akt and AMPK, which was reconciled by ALDH2. Levels of Notch1 and STAT3 phosphorylation were dampened by chronic alcohol intake in FVB but not ALDH2 myocardium. Moreover, the γ-secretase Notch inhibitor N\xE2\x80\x90[N-(3,5-difluorophenacetyl)-1-alany1]-S-phenyglycine t-butyl ester exacerbated ethanol-induced cardiomyocyte contractile dysfunction, apoptosis and autophagy. In summary, these findings suggested that ALDH2 elicits cardioprotection against chronic alcohol intake-induced cardiac geometric and functional anomalies by inhibition of autophagy possibly via restoring the Akt-mTOR-STAT3-Notch signalling cascade.
© 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
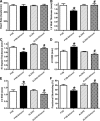
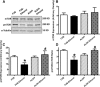
Fig 1
Effect of 12 week alcohol intake on echocardiographic indices in FVB and ALDH2 mice. (A) Heart rate; (B) LV wall thickness; (C) intraventricular (IV) septal thickness; (D) LV EDD; (E) LV ESD and (F) fractional shortening. Mean ± S.E.M., n = 6–11 mice per group, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Fig 2
Effect of 12 week alcohol intake on cardiomyocyte shortening in myocytes from FVB and ALDH2 mice. (A) Resting cell length; (B) peak cell shortening (normalized to cell length); (C) maximal velocity of shortening (+d_L_/d_t_); (D) maximal velocity of relengthening (–d_L_/d_t_); (E) TPS and (F) TR90. Mean ± S.E.M., n = 66–67 cells from three mice per group, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
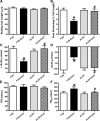
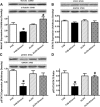
Fig 3
Effect of 12 week alcohol intake on autophagy markers in myocardium from FVB and ALDH2 mice. (A) Beclin-1; (B) LC3B-II; (C) LC3B-I and (D) LC3B-II-to-LC3B-I ratio. Insets: representative immunoblots of Belin-1, LC3B-I, LC3B-II and GAPDH (loading control) using specific antibodies. Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Fig 4
Effect of 12 week alcohol intake on Akt and AMPK signalling in myocardium from FVB and ALDH2 mice. (A) Akt; (B) AMPK; (C) phosphorylated Akt (pAkt); (D) Phosphorylated AMPK (pAMPK); (E) pAkt-to-Akt ratio and (F) pAMPK-to-AMPK ratio. Insets: representative gel blots of pan and pAkt and AMPK using specific antibodies (GAPDH as loading control). Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
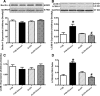
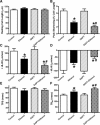
Fig 5
Effect of 12 week alcohol intake on mTOR signalling in myocardium from FVB and ALDH2 mice. (A) Representative gel blots depicting pan and phosphorylated mTOR as well as α-tubulin proteins using specific antibodies; (B) mTOR; (C) pmTOR and (D) pmTOR-to-mTOR ratio. Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
Fig 6
Effect of 12 week alcohol intake on Notch1 and STAT3 signalling in myocardium from FVB and ALDH2 mice. (A) Notch1; (B) STAT3; (C) phosphorylated STAT3 (pSTAT) and (D) pSTAT-to-STAT ratio. Representative gel blots depicting Notch1, STAT3, pSTAT3, α-tubulin and GAPDH (loading controls) using specific antibodies; Mean ± S.E.M., n = 4–5, *P < 0.05 versus FVB group, #P < 0.05 versus FVB-ethanol group.
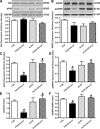
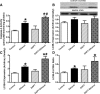
Fig 7
Effect of the Notch inhibitor DAPT on ethanol toxicity-induced cardiomyocyte contractile dysfunction. Cardiomyocytes from FVB mice were incubated with ethanol (240 mg/dl) for four hrs in the absence and presence of DAPT (20 μM) before mechanical function was assessed. (A) Resting cell length; (B) peak cell shortening (normalized to cell length); (C) maximal velocity of shortening (+d_L_/d_t_); (D) maximal velocity of relengthening (–d_L_/d_t_); (E) TPS and (F) TR90. Mean ± S.E.M., n = 46 cells per group, *P < 0.05 versus control group, #P < 0.05 versus ethanol group.
Fig 8
Effect of the Notch inhibitor DAPT on ethanol-induced apoptosis and autophagy. Cardiomyocytes from FVB mice were incubated with ethanol (240 mg/dl) for four hrs in the absence and presence of DAPT (20 μM) prior to assessment of apoptosis and autophagy. (A) Caspase-3 activity; (B) LC3B-1 expression; (C) LC3B-II expression and (D) LC3B-II-to-LC3B-I ratio. Insets: representative gel blots of LC3B-I, LC3B-II and GAPDH (loading control) using specific antibodies. Mean ± S.E.M., n = 3–4 mice per group, *P < 0.05 versus control, #P < 0.05 versus ethanol group.
Similar articles
- AMP-dependent kinase and autophagic flux are involved in aldehyde dehydrogenase-2-induced protection against cardiac toxicity of ethanol.
Ge W, Guo R, Ren J. Ge W, et al. Free Radic Biol Med. 2011 Nov 1;51(9):1736-48. doi: 10.1016/j.freeradbiomed.2011.08.002. Epub 2011 Aug 10. Free Radic Biol Med. 2011. PMID: 21871561 Free PMC article. - A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type i diabetes-induced cardiac dysfunction: role of AMPK-regulated autophagy.
Guo Y, Yu W, Sun D, Wang J, Li C, Zhang R, Babcock SA, Li Y, Liu M, Ma M, Shen M, Zeng C, Li N, He W, Zou Q, Zhang Y, Wang H. Guo Y, et al. Biochim Biophys Acta. 2015 Feb;1852(2):319-31. doi: 10.1016/j.bbadis.2014.05.017. Epub 2014 May 27. Biochim Biophys Acta. 2015. PMID: 24874076 - Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3β and mitochondrial function.
Zhang Y, Babcock SA, Hu N, Maris JR, Wang H, Ren J. Zhang Y, et al. BMC Med. 2012 Apr 23;10:40. doi: 10.1186/1741-7015-10-40. BMC Med. 2012. PMID: 22524197 Free PMC article. Retracted. - ALDH2 in alcoholic heart diseases: molecular mechanism and clinical implications.
Zhang Y, Ren J. Zhang Y, et al. Pharmacol Ther. 2011 Oct;132(1):86-95. doi: 10.1016/j.pharmthera.2011.05.008. Epub 2011 Jun 12. Pharmacol Ther. 2011. PMID: 21664374 Free PMC article. Review. - AKT signalling in the failing heart.
Chaanine AH, Hajjar RJ. Chaanine AH, et al. Eur J Heart Fail. 2011 Aug;13(8):825-9. doi: 10.1093/eurjhf/hfr080. Epub 2011 Jun 30. Eur J Heart Fail. 2011. PMID: 21724622 Free PMC article. Review.
Cited by
- MicroRNA-451 Inhibits Migration of Glioblastoma while Making It More Susceptible to Conventional Therapy.
Ogawa D, Ansari K, Nowicki MO, Salińska E, Bronisz A, Godlewski J. Ogawa D, et al. Noncoding RNA. 2019 Mar 15;5(1):25. doi: 10.3390/ncrna5010025. Noncoding RNA. 2019. PMID: 30875963 Free PMC article. - Enhanced mitophagy in Sertoli cells of ethanol-treated rats: morphological evidence and clinical relevance.
Eid N, Ito Y, Otsuki Y. Eid N, et al. J Mol Histol. 2012 Feb;43(1):71-80. doi: 10.1007/s10735-011-9372-0. Epub 2011 Nov 11. J Mol Histol. 2012. PMID: 22076330 - Alcoholic Cardiomyopathy: Disrupted Protein Balance and Impaired Cardiomyocyte Contractility.
Steiner JL, Lang CH. Steiner JL, et al. Alcohol Clin Exp Res. 2017 Aug;41(8):1392-1401. doi: 10.1111/acer.13405. Epub 2017 May 29. Alcohol Clin Exp Res. 2017. PMID: 28425109 Free PMC article. Review. - Notch signaling and cardiac repair.
Gude N, Sussman M. Gude N, et al. J Mol Cell Cardiol. 2012 Jun;52(6):1226-32. doi: 10.1016/j.yjmcc.2012.03.007. Epub 2012 Mar 21. J Mol Cell Cardiol. 2012. PMID: 22465038 Free PMC article. Review. - Effects of Cardiovascular Risk Factors on Cardiac STAT3.
Pipicz M, Demján V, Sárközy M, Csont T. Pipicz M, et al. Int J Mol Sci. 2018 Nov 12;19(11):3572. doi: 10.3390/ijms19113572. Int J Mol Sci. 2018. PMID: 30424579 Free PMC article. Review.
References
- Fernandez-Sola J, Estruch R, Grau JM, et al. The relation of alcoholic myopathy to cardiomyopathy. Ann Intern Med. 1994;120:529–36. - PubMed
- Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. - PubMed
- Li SY, Gomelsky M, Duan J, et al. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. The Journal of biological chemistry. 2004;279:11244–52. - PubMed
- Ma H, Li J, Gao F, et al. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54:2187–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA013412-02/AA/NIAAA NIH HHS/United States
- R01 AA013412/AA/NIAAA NIH HHS/United States
- R01 AA013412-03/AA/NIAAA NIH HHS/United States
- R01 AA013412-04/AA/NIAAA NIH HHS/United States
- 1R01 AA013412/AA/NIAAA NIH HHS/United States
- R01 AA013412-05/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous