Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain - PubMed (original) (raw)
Review
Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain
Rebecca A Ihrie et al. Neuron. 2011.
Abstract
New neurons and glial cells are generated in an extensive germinal niche adjacent to the walls of the lateral ventricles in the adult brain. The primary progenitors (B1 cells) have astroglial characteristics but retain important neuroepithelial properties. Recent work shows how B1 cells contact all major compartments of this niche. They share the "shoreline" on the ventricles with ependymal cells, forming a unique adult ventricular zone (VZ). In the subventricular zone (SVZ), B1 cells contact transit amplifying (type C) cells, chains of young neurons (A cells), and blood vessels. How signals from these compartments influence the behavior of B1 or C cells remains largely unknown, but recent work highlights growth factors, neurotransmitters, morphogens, and the extracellular matrix as key regulators of this niche. The integration of emerging molecular and anatomical clues forecasts an exciting new understanding of how the germ of youth is actively maintained in the adult brain.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1. The Periventricular Adult Stem Cell Niche
This illustration summarizes recent advances on our understanding of the adult VZ-SVZ niche. The apical ventricular zone is shown at top. Ependymal cells (E, in gray) are multiciliated, and the basal bodies of these cilia are oriented in the direction of cerebrospinal fluid (CSF) flow. Ependymal cells form pinwheel-like structures around the apical processes of type B1 cells (shown in blue). Type B1 cells extend a short, non-motile primary cilium into the ventricle. These cells maintain contact with the ventricle, but disassemble the primary cilium, while dividing. Type B1 cells also frequently extend a basal process with an endfoot that contacts blood vessels (Bv). Type B2 cells, in contrast, have astrocytic characteristics but do not contact the ventricle. Transit-amplifying type C cells (in green) are found close to type B cells. Dividing C cells are also often found in close proximity to blood vessels (shown at left). Type B1 cells also contact their more differentiated progeny, the chains of migrating type A neuroblasts (shown in red). Type A cells migrate tangentially in chains (shown towards right of figure) that ultimately coalesce to form the rostral migratory stream taking these young neurons to the olfactory bulb for terminal differentiation. The VZ-SVZ also includes extracellular matrix (shaded) that contacts all the cell types in this region, including blood vessels and microglia (in purple).
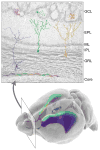
Figure 2. The Adult VZ-SVZ Niche is Patterned and Heterogeneous
A coronal section of the mouse olfactory bulb is shown at top. Neuroblasts derived from the VZ-SVZ enter at the core of the olfactory bulb (at bottom of image), then migrate radially to populate the granular layer (GRL) and glomerular layer (GCL) of the olfactory bulb. Color gradients in the mouse brain depicted at bottom indicate the sites of origin of the differently colored olfactory neurons. The ventral VZ-SVZ principally generates deep granule cells (purple) and calbindin-positive periglomerular cells (magenta). Deep granule cells typically have cell bodies close to the core of the olfactory bulb and dendritic projections that contact the inner half of the external plexiform layer (EPL), close to the mitral cell layer (ML). The dorsal SVZ, by contrast, produces superficial granule cells (green) and tyrosine hydroxylase-positive periglomerular cells (teal). Finally, the medial face of the VZ-SVZ generates calretinin-positive superficial granule cells (yellow) and periglomerular cells (orange). Note that periglomerular cells tend to be produced in more anterior regions of the VZ-SVZ, as indicated by the gradients shown in the lower image.
Similar articles
- Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain.
Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Mirzadeh Z, et al. Cell Stem Cell. 2008 Sep 11;3(3):265-78. doi: 10.1016/j.stem.2008.07.004. Cell Stem Cell. 2008. PMID: 18786414 Free PMC article. - A specialized vascular niche for adult neural stem cells.
Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. Tavazoie M, et al. Cell Stem Cell. 2008 Sep 11;3(3):279-88. doi: 10.1016/j.stem.2008.07.025. Cell Stem Cell. 2008. PMID: 18786415 Free PMC article. - Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment.
Tramontin AD, García-Verdugo JM, Lim DA, Alvarez-Buylla A. Tramontin AD, et al. Cereb Cortex. 2003 Jun;13(6):580-7. doi: 10.1093/cercor/13.6.580. Cereb Cortex. 2003. PMID: 12764031 Review. - Persistent Cyfip1 Expression Is Required to Maintain the Adult Subventricular Zone Neurogenic Niche.
Habela CW, Yoon KJ, Kim NS, Taga A, Bell K, Bergles DE, Maragakis NJ, Ming GL, Song H. Habela CW, et al. J Neurosci. 2020 Mar 4;40(10):2015-2024. doi: 10.1523/JNEUROSCI.2249-19.2020. Epub 2020 Jan 27. J Neurosci. 2020. PMID: 31988061 Free PMC article. - The subventricular zone: new molecular and cellular developments.
Conover JC, Allen RL. Conover JC, et al. Cell Mol Life Sci. 2002 Dec;59(12):2128-35. doi: 10.1007/s000180200012. Cell Mol Life Sci. 2002. PMID: 12568338 Free PMC article. Review.
Cited by
- Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4.
Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Benner EJ, et al. Nature. 2013 May 16;497(7449):369-73. doi: 10.1038/nature12069. Epub 2013 Apr 24. Nature. 2013. PMID: 23615612 Free PMC article. - Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis.
Kawaguchi D, Furutachi S, Kawai H, Hozumi K, Gotoh Y. Kawaguchi D, et al. Nat Commun. 2013;4:1880. doi: 10.1038/ncomms2895. Nat Commun. 2013. PMID: 23695674 Free PMC article. - Holding on to stemness.
Lathia JD, Rich JN. Lathia JD, et al. Nat Cell Biol. 2012 May 2;14(5):450-2. doi: 10.1038/ncb2498. Nat Cell Biol. 2012. PMID: 22552145 - Dephosphorylation of 4EBP1/2 Induces Prenatal Neural Stem Cell Quiescence.
Geben LC, Brockman AA, Chalkley MBL, Sweet SR, Gallagher JE, Scheuing AL, Simerly RB, Ess KC, Irish JM, Ihrie RA. Geben LC, et al. bioRxiv [Preprint]. 2023 Feb 15:2023.02.14.528513. doi: 10.1101/2023.02.14.528513. bioRxiv. 2023. PMID: 36824760 Free PMC article. Preprint. - Restricted nature of adult neural stem cells: re-evaluation of their potential for brain repair.
Obernier K, Tong CK, Alvarez-Buylla A. Obernier K, et al. Front Neurosci. 2014 Jun 17;8:162. doi: 10.3389/fnins.2014.00162. eCollection 2014. Front Neurosci. 2014. PMID: 24987325 Free PMC article.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. - PubMed
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007:990–1002. - PubMed
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 HD032116-17/HD/NICHD NIH HHS/United States
- NS28478/NS/NINDS NIH HHS/United States
- R37 HD032116-18/HD/NICHD NIH HHS/United States
- HD32116/HD/NICHD NIH HHS/United States
- R01 NS028478-19/NS/NINDS NIH HHS/United States
- R37 HD032116/HD/NICHD NIH HHS/United States
- R37 NS028478/NS/NINDS NIH HHS/United States
- R01 NS028478-20/NS/NINDS NIH HHS/United States
- R01 NS028478/NS/NINDS NIH HHS/United States
- R01 HD032116/HD/NICHD NIH HHS/United States
- R37 HD032116-16/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources