Transient expression of Mnb/Dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling - PubMed (original) (raw)
Transient expression of Mnb/Dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling
Barbara Hämmerle et al. Development. 2011 Jun.
Abstract
The decision of a neural precursor to stop dividing and begin its terminal differentiation at the correct place, and at the right time, is a crucial step in the generation of cell diversity in the nervous system. Here, we show that the Down's syndrome candidate gene (Mnb/Dyrk1a) is transiently expressed in prospective neurons of vertebrate CNS neuroepithelia. The gain of function (GoF) of Mnb/Dyrk1a induced proliferation arrest. Conversely, its loss of function (LoF) caused over proliferation and cell death. We found that MNB/DYRK1A is both necessary and sufficient to upregulate, at transcriptional level, the expression of the cyclin-dependent kinase inhibitor p27(KIP1) in the embryonic chick spinal cord and mouse telencephalon, supporting a regulatory role for MNB/DYRK1A in cell cycle exit of vertebrate CNS neurons. All these actions required the kinase activity of MNB/DYRK1A. We also observed that MNB/DYRK1A is co-expressed with the NOTCH ligand Delta1 in single neuronal precursors. Furthermore, we found that MNB/DYRK1A suppressed NOTCH signaling, counteracted the pro-proliferative action of the NOTCH intracellular domain (NICD), stimulated Delta1 expression and was required for the neuronal differentiation induced by the decrease in NOTCH signaling. Nevertheless, although Mnb/Dyrk1a GoF led to extensive withdrawal of neuronal precursors from the cell cycle, it was insufficient to elicit their differentiation. Remarkably, a transient (ON/OFF) Mnb/Dyrk1a GoF efficiently induced neuronal differentiation. We propose that the transient expression of MNB/DYRK1A in neuronal precursors acts as a binary switch, coupling the end of proliferation and the initiation of neuronal differentiation by upregulating p27KIP1 expression and suppressing NOTCH signaling.
Figures
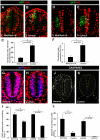
Fig. 1.
Effect of the gain and loss of MNB/DYRK1A function on cell proliferation in the chick spinal cord. All images were collected from transverse vibratome sections taken at the level of the rostral spinal cord (NZ) of HH13-16 chick embryos. (A,B) Single confocal sections of embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls), and immunostained for BrdU and GFP. Arrows indicate BrdU/GFP double-labeled cells. (D,E) Confocal projections (50 μm) of embryos transfected as above showing immunolabeling for PH3 and GFP. Arrows indicate PH3/GFP double-labeled cells. (G,H,J,K) Confocal projections (9 μm) of embryos cultured in the presence or absence (controls) of harmine (as indicated) showing BrdU (G,H) and activated caspase 3 (J,K) immunostaining. Nuclei were counterstained with DAPI. (C,F,I,L) Statistical analysis of the experiments as indicated. Data are mean±s.e.m.
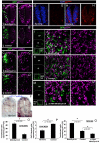
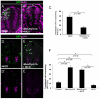
Fig. 2.
Effects of loss and gain of MNB/DYRK1A function on p27KIP1 expression in the chick spinal cord and mouse telencephalon. (A-D′) All images were collected from transverse vibratome sections taken at the level of the rostral spinal cord (NZ) of HH13-16 chick embryos. (A-B′) Confocal projections (10 μm) of embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls), and immunolabeled for p27KIP1 and GFP 18 hours after electroporation. Note the appearance of p27KIP1-positive cells in the transfected zone (outlined) in A. (C-D′) Confocal projections (15 μm) of embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls), and immunolabeled for GFP after fluorescent in situ hybridization for p27KIP1, 12 hours after electroporation. (E-H′) Embryos cultured in the presence or absence (controls) of harmine for 6 hours and analyzed for the expression of p27KIP1 mRNA by in situ hybridization (E,F) or for protein (G-H′). Note the decrease in the number of p27KIP1-positive cells in harmine-treated embryos. (I,K,M) Coronal confocal sections showing GFP-positive cells at the frontal area of developing cerebral cortex of E14.5 embryos electroporated with pCIG-Mnb/Dyrk1a (Mnb/Dyrk1a GoF), pCIG (control) or Mnb/Dyrk1a siRNA + pCIG (Mnb/Dyrk1a LoF) and cultured afterwards for 24 hours. (J,J′,L,L′,N,N′) High-magnification images taken from the VZ in the boxed areas indicated in I,K,M showing p27KIP1 and GFP immunolabeling. Double GFP/p27KIP1-labeled cells are indicated with arrows. CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. (O-Q) Statistical analysis of the experiments as indicated. Data are mean±s.e.m.
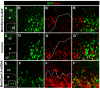
Fig. 3.
Effect of MNB/DYRK1A on DELTA-NOTCH signaling. (A) Confocal projection of the prospective spinal cord of a HH10 whole-mount chick embryo (dorsal view) showing double fluorescent in situ hybridization labeling of Mnb/Dyrk1a and Delta1 in the neural tube, as well as some in migrating neural crest (NC) cells (arrows). (B-B″) Single confocal image at higher magnification of the boxed region at the level of somites 1-2 showing cellular co-labeling in the rostral spinal cord. (C-F′) Confocal projections (10 μM) of HH15-16 chick embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls) showing fluorescent in situ hybridization labeling for either HES5 or DELTA1 and GFP inmmunolabeling 15 hours after electroporation. (Ca-Fa′) Single confocal image at higher magnification of the boxed regions showing double-labeled cells (arrows). (G,H) Statistical analysis of the corresponding experiments as indicated. Data are mean±s.e.m.
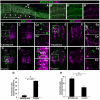
Fig. 4.
Effects of the loss and gain of Mnb/Dyrk1a function on Hes5 expression in the mouse telencephalon. (A,C,E) Coronal confocal sections showing GFP-expressing cells at the frontal area of the developing cerebral cortex of E14.5 embryos electroporated with pCIG-Mnb/Dyrk1a (Mnb/Dyrk1a GoF), pCIG (control) or Mnb/Dyrk1a siRNA + pCIG (Mnb/Dyrk1a LoF), and cultured for 24 hours afterwards. (B-B″,D-D″,F-F″) High-magnification images taken from the boxed areas indicated in A,C,E showing fluorescent in situ hybridization labeling for Hes5 and GFP immunolabeling. Note the decrease in the number of _Hes5_-positive cells within the transfected region (dotted line) after Mnb/Dyrk1a GoF (B-B″), and the increase of Hes5 expression after Mnb/Dyrk1a LoF (F-F″), when compared with the control (D-D″) and with the neighboring non-transfected areas. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone.
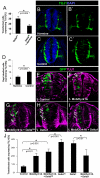
Fig. 5.
The effects of the interaction of MNB/DYRK1A with NOTCH signaling on neural proliferation. (A,B) Confocal projections (10 μm) showing immunolabeling for BrdU and GFP at the level of the spinal cord NZ of HH15-16 chick embryos that were transfected with pEVRF-NICD or pCIG-Mnb/Dyrk1a + pEVRF-NICD. (C) Statistical analysis of the results from this experiment. Although NICD strongly suppresses neuronal differentiation in the spinal cord (Hämmerle and Tejedor, 2007), the proportion of control (pCIG) transfected cells that differentiate at this stage is rather low (7%, control in Fig. 6J) and, accordingly, NICD produced only a slight increase of BrdU incorporation over control transfected cells (compare with control in Fig. 1C). Data are mean±s.e.m. (D,E) Confocal projections (10 μm) showing immunolabeling for p27KIP1 and GFP at the level of the spinal cord NZ of HH15-16 chick embryos that were transfected with pEVRF-NICD or pCIG-Mnb/Dyrk1a + pEVRF-NICD. (F) Statistical analysis of the effect on p27KIP1 expression in the spinal cord NZ of HH15-16 chick embryos transfected with: pCIG (control); pCIG-Mnb/Dyrk1a; pCIG-Mnb/Dyrk1a + pEVRF-NICD; pEVRF-NICD. Both MNB/DYRK1A alone or with NICD greatly increases the proportion of p27KIP1-positive cells, whereas NICD alone significantly decreases the number of cells expressing p27KIP1 compared with the control. Data are mean±s.e.m.
Fig. 6.
Effects of MNB/DYRK1A LoF or GoF on neuronal differentiation induced by the suppression of NOTCH signaling. (A) HH11-12 embryos were transfected with pCIG-DeltaDN in the NZ and, after 3 hours in ovo, embryos were cultured in the presence or absence of harmine for 15 hours. Data are mean±s.e.m. (B-C′) Representative images showing TUJ1 immunolabeling and DAPI counterstaining in sections of the rostral spinal cord from HH11-12 embryos that were cultured for 12 hours in the presence or absence of harmine. (D) Quantitative analysis of this experiment. Data are mean±s.e.m. (E-I) Representative images showing TUJ1 immunolabeling and GFP in sections of the rostral spinal cord from HH11-12 embryos that were transfected with the pCIG vector carrying the cDNAs indicated, and analyzed 18 hours afterwards. Arrows indicate the transfected cells expressing TUJ1. (J) Statistical analysis of the effect of the indicated constructs on TUJ1 expression in the NZ. Data are mean±s.e.m.
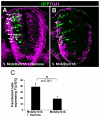
Fig. 7.
Effect of the transient (ON/OFF) MNB/DYRK1A GoF on neuronal differentiation. (A,B) Confocal projections (10 μm) of transverse sections from the spinal cord NZ in HH11-12 chick embryos that were transfected with pCIG-Mnb/Dyrk1a and, after 24 hours in ovo, cultured for 6 hours in the presence or absence of harmine as indicated. Arrows indicate GFP/TUJ1 double-labeled cells. (C) Statistical analysis of the data from this experiment. Note the important increase in the ratio TUJ1-positive cells that was achieved when MNB/DYRK1A transfection was followed by harmine treatment. Data are mean±s.e.m.
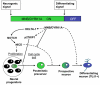
Fig. 8.
A working model: MNB/DYRK1A functions as a binary switch in the coupling of cell cycle exit and neuronal differentiation. During neurogenesis, NOTCH signaling maintains neural progenitors in proliferation and inhibits neuronal differentiation. MNB/DYRK1A is transiently (ON/OFF) expressed in single neuronal precursors. The upregulation of MNB/DYRK1A expression (ON phase, presumably caused by neurogenic signals) promotes cell cycle exit by upregulating p27KIP1 expression. In addition, MNB/DYRK1A suppresses NOTCH signaling and upregulates Delta1 expression, which increases NOTCH signaling in the neighboring cells and reinforces the feedback loop of lateral inhibition, thereby facilitating the generation of prospective neurons that remain in a quiescent state while MNB/DYRK1A expression level is high. Subsequently, MNB/DYRK1A is downregulated (OFF phase, presumably caused by differentiating signals), allowing the prospective neuron to differentiate.
Similar articles
- Mnb/Dyrk1A orchestrates a transcriptional network at the transition from self-renewing neurogenic progenitors to postmitotic neuronal precursors.
Shaikh MN, Tejedor FJ. Shaikh MN, et al. J Neurogenet. 2018 Mar;32(1):37-50. doi: 10.1080/01677063.2018.1438427. J Neurogenet. 2018. PMID: 29495936 - Minibrain drives the Dacapo-dependent cell cycle exit of neurons in the Drosophila brain by promoting asense and prospero expression.
Shaikh MN, Gutierrez-Aviño F, Colonques J, Ceron J, Hämmerle B, Tejedor FJ. Shaikh MN, et al. Development. 2016 Sep 1;143(17):3195-205. doi: 10.1242/dev.134338. Epub 2016 Aug 10. Development. 2016. PMID: 27510975 - The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation.
Soppa U, Schumacher J, Florencio Ortiz V, Pasqualon T, Tejedor FJ, Becker W. Soppa U, et al. Cell Cycle. 2014;13(13):2084-100. doi: 10.4161/cc.29104. Epub 2014 May 7. Cell Cycle. 2014. PMID: 24806449 Free PMC article. - The MNB/DYRK1A protein kinase: neurobiological functions and Down syndrome implications.
Hämmerle B, Elizalde C, Galceran J, Becker W, Tejedor FJ. Hämmerle B, et al. J Neural Transm Suppl. 2003;(67):129-37. doi: 10.1007/978-3-7091-6721-2_11. J Neural Transm Suppl. 2003. PMID: 15068245 Review. - The MNB/DYRK1A protein kinase: genetic and biochemical properties.
Galceran J, de Graaf K, Tejedor FJ, Becker W. Galceran J, et al. J Neural Transm Suppl. 2003;(67):139-48. doi: 10.1007/978-3-7091-6721-2_12. J Neural Transm Suppl. 2003. PMID: 15068246 Review.
Cited by
- DYRK1A interacts with the tuberous sclerosis complex and promotes mTORC1 activity.
Wang P, Sarkar S, Zhang M, Xiao T, Kong F, Zhang Z, Balasubramanian D, Jayaram N, Datta S, He R, Wu P, Chao P, Zhang Y, Washburn M, Florens LA, Nagarkar-Jaiswal S, Jaiswal M, Mohan M. Wang P, et al. Elife. 2024 Oct 22;12:RP88318. doi: 10.7554/eLife.88318. Elife. 2024. PMID: 39436397 Free PMC article. - Neurobiological research on N,N-dimethyltryptamine (DMT) and its potentiation by monoamine oxidase (MAO) inhibition: from ayahuasca to synthetic combinations of DMT and MAO inhibitors.
Egger K, Aicher HD, Cumming P, Scheidegger M. Egger K, et al. Cell Mol Life Sci. 2024 Sep 10;81(1):395. doi: 10.1007/s00018-024-05353-6. Cell Mol Life Sci. 2024. PMID: 39254764 Free PMC article. Review. - Sex-specific developmental alterations in DYRK1A expression in the brain of a Down syndrome mouse model.
Hawley LE, Stringer M, Deal AJ, Folz A, Goodlett CR, Roper RJ. Hawley LE, et al. Neurobiol Dis. 2024 Jan;190:106359. doi: 10.1016/j.nbd.2023.106359. Epub 2023 Nov 20. Neurobiol Dis. 2024. PMID: 37992782 Free PMC article. - Regulation of CMGC kinases by hypoxia.
Kim K, Lee SB. Kim K, et al. BMB Rep. 2023 Nov;56(11):584-593. doi: 10.5483/BMBRep.2023-0165. BMB Rep. 2023. PMID: 37915135 Free PMC article. Review. - The mitochondrial ribosomal protein mRpL4 regulates Notch signaling.
Mo D, Liu C, Chen Y, Cheng X, Shen J, Zhao L, Zhang J. Mo D, et al. EMBO Rep. 2023 Jun 5;24(6):e55764. doi: 10.15252/embr.202255764. Epub 2023 Apr 3. EMBO Rep. 2023. PMID: 37009823 Free PMC article.
References
- Adayev T., Chen-Hwang M. C., Murakami N., Wegiel J., Hwang Y. W. (2006). Kinetic properties of a MNB⁄DYRK1A mutant suitable for the elucidation of biochemical pathways. Biochemistry 45, 12011-12019. - PubMed
- Agathocleous M., Harris W. A.(2009). From progenitors to differentiated cells in the vertebrate retina. Annu. Rev. Cell Dev. Biol. 25, 45-69. - PubMed
- Akazawa C., Sasai Y., Nakanishi S., Kageyama R. (1992). Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J. Biol. Chem. 267, 21879-21885. - PubMed
- Altafaj X., Dierssen M., Baamonde C., Martí E., Visa J., Guimerà J., Oset M., González J. R., Flórez J., Fillat C., et al. (2001) Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Hum. Mol. Genet. 10, 1915-1923 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous