Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area - PubMed (original) (raw)
Comparative Study
Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area
Yanfang Xia et al. J Neurosci. 2011.
Abstract
The midbrain ventral tegmental area (VTA) projection to the nucleus accumbens (NAc) is implicated in motivation and reinforcement. A significant number of NAc medium spiny neurons (MSNs) project back to the VTA, although the nature of this projection is essentially unknown. For example, do NAc MSNs directly target accumbens-projecting dopamine neurons and do they act via the GABA(A) or GABA(B) receptor? To address these issues, we expressed the light-sensitive channel rhodopsin-2 in the rat NAc and made electrophysiological recordings from VTA neurons ex vivo. We found that the NAc directly targets non-dopaminergic VTA neurons, including some that project back to the NAc. These MSN GABAergic terminals are opioid sensitive and act via GABA(A) receptors.
Figures
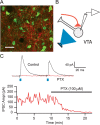
Figure 1.
ChR2-expression in the NAc. A, Confocal image of ChR2–tdtomato expression (red) in NAc neurons. Scale bar, 50 ìm. B, Diagram of experimental design: light stimulation (blue) of ChR2 expressing NAc MSN (red). C, Example recording from an MSN showing responses to 1 ms pulses of light at three different intensities. D, MSNs can follow trains of light pulses up to 100 Hz. E, Average probability of firing an action potential (AP) for each stimulus in a train for three different frequencies (n = 6). F, Example recording of a light-evoked IPSC in a neighboring uninfected MSN (current is inward when holding MSN at −75 mV). This IPSC was entirely blocked after the application of picrotoxin (PTX). G, IPSCs evoked in response to a 50 Hz train of light.
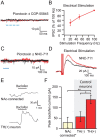
Figure 2.
ChR2-expressing MSNs project to the VTA. A, Confocal image of ChR2–tdtomato expression (red) in axon terminals within the VTA near TH+ neurons (green) after microinjection into the NAc. Scale bar, 50 ìm. B, Diagram of experimental design: light stimulates ChR2-expressing NAc MSN terminals (red) while recording from uninfected VTA neurons. C, Example recording from VTA neuron showing responses to two 3 ms pulses of light, 50 ms apart. This IPSC was entirely blocked after application of picrotoxin (PTX). Bottom graph shows time course of pharmacological effect.
Figure 3.
Lack of light-evoked GABAB response in VTA neurons. A, Average of five consecutive traces in an NAc-connected neuron after the GABAA response was blocked with 100 μ
m
picrotoxin and after application of the GABAB receptor antagonist CGP-55845 [(2_S_)-3-[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid] shows no response to 100 Hz light stimulation. B, The amplitude of electrically evoked GABAB IPSCs depends on the stimulation frequency. C, The addition of NNC-711 (10 μ
m
) does not reveal a light-evoked GABAB IPSC. D, NNC-711 enhanced electrically evoked GABAB IPSCs. E, Bath application of 20 μ
m
baclofen produced a small outward current in a neuron that received input from the NAc (top) compared with the response of a control TH (+) neuron (bottom). F, Connected neurons (n = 5) showed significantly smaller baclofen responses than TH (+) control neurons (n = 9), which had larger responses to baclofen than identified TH (−) neurons (n = 5). *p < 0.05, **p < 0.01.
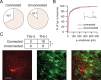
Figure 4.
NAc MSNs target VTA non-dopamine neurons. A, Proportion of connected and unconnected neurons that express the _I_h. B, Among _I_h-expressing neurons, there was no difference in the magnitude of the _I_h in connected and unconnected neurons. C, Example of a biocytin-filled TH (−) neuron that responded to light stimulation with an IPSC. Biocytin, Red; TH, green. Inset shows summary of TH immunocytochemistry for connected and unconnected neurons. *p < 0.01.
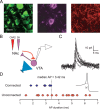
Figure 5.
NAc MSNs target NAc-projecting VTA neurons. A, Triple-labeled VTA slice showing TH (green), DiI (violet), and ChR2 (red) after microinjection of DiI and ChR2 into the NAc. Scale bar, 20 ìm. B, Diagram of experimental design. Light stimulation (blue) of ChR2-expressing NAc MSN terminals (red) while recording from retrogradely labeled VTA neurons (violet). C, Ten consecutive light-evoked IPSCs from a retrogradely labeled VTA neuron. D, Distribution of action potential durations from connected (blue) and unconnected (red) NAc-projecting neurons. Inset shows average of 10 consecutive action potentials (AP; from example in C). Dashed line indicates where action potential duration was measured.
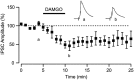
Figure 6.
NAc projections to the VTA are inhibited by μ-opioids. Bath application of the μ-opioid receptor agonist DAMGO (1 μ
m
) inhibited light-evoked IPSCs arising from the NAc (n = 9). Top traces show average of five consecutive IPSCs from control (left) and in DAMGO (right).
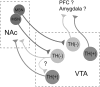
Figure 7.
Circuit diagram of NAc connections within the VTA MSNs of the NAc synapse onto non-dopaminergic neurons in the VTA, including some that project back to the NAc. NAc MSNs do not synapse onto VTA dopamine neurons but may indirectly modulate their activity through local connections within the VTA.
Similar articles
- Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning.
Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Lüscher C. Brown MT, et al. Nature. 2012 Dec 20;492(7429):452-6. doi: 10.1038/nature11657. Epub 2012 Nov 25. Nature. 2012. PMID: 23178810 - The Projection Targets of Medium Spiny Neurons Govern Cocaine-Evoked Synaptic Plasticity in the Nucleus Accumbens.
Baimel C, McGarry LM, Carter AG. Baimel C, et al. Cell Rep. 2019 Aug 27;28(9):2256-2263.e3. doi: 10.1016/j.celrep.2019.07.074. Cell Rep. 2019. PMID: 31461643 Free PMC article. - Differential expression of long-term potentiation among identified inhibitory inputs to dopamine neurons.
Simmons DV, Petko AK, Paladini CA. Simmons DV, et al. J Neurophysiol. 2017 Oct 1;118(4):1998-2008. doi: 10.1152/jn.00270.2017. Epub 2017 Jul 12. J Neurophysiol. 2017. PMID: 28701538 Free PMC article. - Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching.
Mingote S, Amsellem A, Kempf A, Rayport S, Chuhma N. Mingote S, et al. Neurochem Int. 2019 Oct;129:104482. doi: 10.1016/j.neuint.2019.104482. Epub 2019 Jun 3. Neurochem Int. 2019. PMID: 31170424 Free PMC article. Review. - Opioid-induced structural and functional plasticity of medium-spiny neurons in the nucleus accumbens.
Thompson BL, Oscar-Berman M, Kaplan GB. Thompson BL, et al. Neurosci Biobehav Rev. 2021 Jan;120:417-430. doi: 10.1016/j.neubiorev.2020.10.015. Epub 2020 Nov 2. Neurosci Biobehav Rev. 2021. PMID: 33152423 Free PMC article. Review.
Cited by
- Understanding opioid reward.
Fields HL, Margolis EB. Fields HL, et al. Trends Neurosci. 2015 Apr;38(4):217-25. doi: 10.1016/j.tins.2015.01.002. Epub 2015 Jan 29. Trends Neurosci. 2015. PMID: 25637939 Free PMC article. Review. - Establishing causality for dopamine in neural function and behavior with optogenetics.
Steinberg EE, Janak PH. Steinberg EE, et al. Brain Res. 2013 May 20;1511:46-64. doi: 10.1016/j.brainres.2012.09.036. Epub 2012 Sep 29. Brain Res. 2013. PMID: 23031636 Free PMC article. Review. - Functional Magnetic Resonance Imaging of Electrical and Optogenetic Deep Brain Stimulation at the Rat Nucleus Accumbens.
Albaugh DL, Salzwedel A, Van Den Berge N, Gao W, Stuber GD, Shih YY. Albaugh DL, et al. Sci Rep. 2016 Sep 7;6:31613. doi: 10.1038/srep31613. Sci Rep. 2016. PMID: 27601003 Free PMC article. - BEHAVIORAL AND NEUROBIOLOGICAL MECHANISMS OF PAVLOVIAN AND INSTRUMENTAL EXTINCTION LEARNING.
Bouton ME, Maren S, McNally GP. Bouton ME, et al. Physiol Rev. 2021 Apr 1;101(2):611-681. doi: 10.1152/physrev.00016.2020. Epub 2020 Sep 24. Physiol Rev. 2021. PMID: 32970967 Free PMC article. Review. - Nucleus Accumbens Modulation in Reward and Aversion.
Klawonn AM, Malenka RC. Klawonn AM, et al. Cold Spring Harb Symp Quant Biol. 2018;83:119-129. doi: 10.1101/sqb.2018.83.037457. Epub 2019 Jan 23. Cold Spring Harb Symp Quant Biol. 2018. PMID: 30674650 Free PMC article.
References
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. - PubMed
- Bechara A, Harrington F, Nader K, van der Kooy D. Neurobiology of motivation: double dissociation of two motivational mechanisms mediating opiate reward in drug-naive versus drug-dependent animals. Behav Neurosci. 1992;106:798–807. - PubMed
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. - PubMed
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous