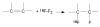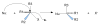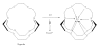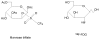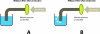Review of F-FDG Synthesis and Quality Control - PubMed (original) (raw)
Review of F-FDG Synthesis and Quality Control
S Yu. Biomed Imaging Interv J. 2006 Oct.
Abstract
This review article covers a concise account on fludeoxyglucose ((18)F-FDG) synthesis and quality control procedures with emphasis on practical synthesis Currently, (18)F-FDG is the most successful PET radiopharmaceutical so far. The advancement in synthesis and quality control of (18)F-FDG, together with its approval by the US FDA and the availability of reimbursement, are probably the main reasons for the flourish of clinical PET over the last 20 years. (18)F-FDG can be synthesised by either electrophilic fluorination or nucleophilic fluorination reaction. Nucleophilic fluorination using mannose triflate as precursor and Kryptofix or tetrabutylammonium salts (TBA) is widely used because of higher yield and shorter reaction time. The quality control requirements of (18)F-FDG can be found in United States Pharmacopeia (USP), British Pharmacopeia (BP), European Pharmacopeia (EP) and the Chemistry, Manufacturing, and Controls (CMC) section from United States Food and Drug Administration (US FDA) PET draft guidance documents. Basic requirements include radionuclidic identity, radiochemical purity, chemical purity, pH, residual solvent, sterility, and bacterial endotoxin level. Some of these tests (sterility, endotoxins and radionuclidic purity) can be finished after the (18)F-FDG has been released. Although USP, BP and EP do not require filter membrane integrity test, many laboratories perform this test as an indirect evident of the product sterility. It is also interesting to note that there are major differences in (18)F-FDG quality requirements among USP, BP, and CMC.
Figures
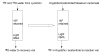
Figure 1
Electrophilic fluorination.
Figure 2
Synthesis of 18F-FDG by electrophilic fluorination.
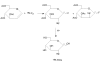
Figure 3
Nucleophilic substitution: Nu = nucleophilic molecule, X = leaving group.
Figure 4
Synthesis of 18F-FDG by nucleophilic substitution.
Figure 5
(A) Retention of 18F-FDG in light QMA ion exchange column (B) elution of 18F from light QMA ion exchange column.
Figure 6
Kryptofix 222 ™ and K+.
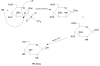
Figure 7
Structures of Mannose triflate and 18F-FDG.
Figure 8
(A): Filter membrane is intact, no air passes through the membrane, no air bubble in water. (B): Filter membrane is broken or at bubble point, air passes through the membrane, air bubble in water. The bubble point should be higher than or equal to maximum pressure listed in the specification of the filter.
Similar articles
- A "dose on demand" Biomarker Generator for automated production of [(18)F]F(-) and [(18)F]FDG.
Awasthi V, Watson J, Gali H, Matlock G, McFarland A, Bailey J, Anzellotti A. Awasthi V, et al. Appl Radiat Isot. 2014 Jul;89:167-75. doi: 10.1016/j.apradiso.2014.02.015. Epub 2014 Mar 6. Appl Radiat Isot. 2014. PMID: 24650573 - Regulation of the compounding of positron emission tomography drugs.
Hung JC. Hung JC. Am J Health Syst Pharm. 2001 Jan 15;58(2):134-9. doi: 10.1093/ajhp/58.2.134. Am J Health Syst Pharm. 2001. PMID: 11202536 Review.
Cited by
- PET Imaging of Fibroblast Activation Protein in Various Types of Cancer Using 68Ga-FAP-2286: Comparison with 18F-FDG and 68Ga-FAPI-46 in a Single-Center, Prospective Study.
Pang Y, Zhao L, Meng T, Xu W, Lin Q, Wu H, Zhang J, Chen X, Sun L, Chen H. Pang Y, et al. J Nucl Med. 2023 Mar;64(3):386-394. doi: 10.2967/jnumed.122.264544. Epub 2022 Sep 2. J Nucl Med. 2023. PMID: 36215571 Free PMC article. - Imaging Inflammation with Positron Emission Tomography.
Iking J, Staniszewska M, Kessler L, Klose JM, Lückerath K, Fendler WP, Herrmann K, Rischpler C. Iking J, et al. Biomedicines. 2021 Feb 19;9(2):212. doi: 10.3390/biomedicines9020212. Biomedicines. 2021. PMID: 33669804 Free PMC article. Review. - Establishing the [18F]-FDG Production via Two Different Automated Synthesizers for Routine Clinical Studies: Our Institutional Experiences of 4 years.
Saxena P, Singh AK, Dixit M, Kheruka SC, Mahmood T, Gambhir S. Saxena P, et al. Indian J Nucl Med. 2021 Apr-Jun;36(2):120-124. doi: 10.4103/ijnm.IJNM_137_20. Epub 2021 Jun 21. Indian J Nucl Med. 2021. PMID: 34385781 Free PMC article. - Focal incidental colorectal fluorodeoxyglucose uptake: Should it be spotlighted?
Lee H, Hwang KH. Lee H, et al. World J Clin Cases. 2024 May 26;12(15):2466-2474. doi: 10.12998/wjcc.v12.i15.2466. World J Clin Cases. 2024. PMID: 38817235 Free PMC article. - Quality Assessment in FDG-PET/CT Imaging of Head-and-Neck Cancer: One Home Run Is Better Than Two Doubles.
Van den Wyngaert T, De Schepper S, Carp L. Van den Wyngaert T, et al. Front Oncol. 2020 Aug 14;10:1458. doi: 10.3389/fonc.2020.01458. eCollection 2020. Front Oncol. 2020. PMID: 32923399 Free PMC article. Review.
References
- Gallagher BM, Fowler JS, Gutterson NI, et al. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19(10):1154–61. - PubMed
- Silverman M, Aganon MA, Chinard FP. Specificity of monosaccharide transport in dog kidney. Am J Physiol. 1970;218(3):743–50. - PubMed
- Bessell EM, Courtenay VD, Foster AB, et al. Some in vivo and in vitro antitumour effects of the deoxyfluoro-D-glucopyranoses. Eur J Cancer. 1973;9(7):463–70. - PubMed
- Fowler JS, Ido T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med. 2002;32(1):6–12. - PubMed
- Ehrenkaufer RE, Potocki JF, Jewett DM. Simple synthesis of F-18-labeled 2-fluoro-2-deoxy-D-glucose: concise communication. J Nucl Med. 1984;25(3):333–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources