Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain - PubMed (original) (raw)
. 2011 Jun;134(Pt 6):1777-89.
doi: 10.1093/brain/awr094.
Nobutaka Horie, William Slikker, Hadar Keren-Gill, Ke Zhan, Guohua Sun, Nathan C Manley, Marta P Pereira, Lamiya A Sheikh, Erin L McMillan, Bruce T Schaar, Clive N Svendsen, Tonya M Bliss, Gary K Steinberg
Affiliations
- PMID: 21616972
- PMCID: PMC3102243
- DOI: 10.1093/brain/awr094
Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain
Robert H Andres et al. Brain. 2011 Jun.
Abstract
Stem cell transplantation promises new hope for the treatment of stroke although significant questions remain about how the grafted cells elicit their effects. One hypothesis is that transplanted stem cells enhance endogenous repair mechanisms activated after cerebral ischaemia. Recognizing that bilateral reorganization of surviving circuits is associated with recovery after stroke, we investigated the ability of transplanted human neural progenitor cells to enhance this structural plasticity. Our results show the first evidence that human neural progenitor cell treatment can significantly increase dendritic plasticity in both the ipsi- and contralesional cortex and this coincides with stem cell-induced functional recovery. Moreover, stem cell-grafted rats demonstrated increased corticocortical, corticostriatal, corticothalamic and corticospinal axonal rewiring from the contralesional side; with the transcallosal and corticospinal axonal sprouting correlating with functional recovery. Furthermore, we demonstrate that axonal transport, which is critical for both proper axonal function and axonal sprouting, is inhibited by stroke and that this is rescued by the stem cell treatment, thus identifying another novel potential mechanism of action of transplanted cells. Finally, we established in vitro co-culture assays in which these stem cells mimicked the effects observed in vivo. Through immunodepletion studies, we identified vascular endothelial growth factor, thrombospondins 1 and 2, and slit as mediators partially responsible for stem cell-induced effects on dendritic sprouting, axonal plasticity and axonal transport in vitro. Thus, we postulate that human neural progenitor cells aid recovery after stroke through secretion of factors that enhance brain repair and plasticity.
Figures
Figure 1
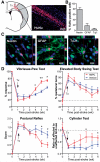
Differentiation fate of transplanted human NPCs and effect on behavioural recovery after stroke. (A) Human NPCs (human nuclear antigen+ cells; red) exhibit good survival and migration towards the lesion at 5 weeks post-transplantation. Representative photomicrograph of human NPCs in the peri-infarct region. Grey-shaded area indicates the lesion. (B) Differentiation profile of the human NPCs at 5 weeks post-transplant. (C) Confocal images of differentiation markers co-localizing with human nuclear antigen in the peri-infarct area. (D) Human NPC-grafted animals have significantly improved functional recovery compared with vehicle-injected controls in three out of four behaviour tests; *P < 0.05, **P < 0.01. n = 12 per group, except cylinder test n = 6. Scale bars: A = 50 µm, C = 10 µm. GFAP = glial fibrillary acidic protein; HuNu = human nuclear antigen; TuJ1 = neuronal class III β-Tubulin; Tx = transplantation.
Figure 2
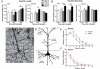
Human NPC transplantation enhances dendritic plasticity after stroke. (A and B) Human NPCs enhance dendritic length (A) and branching (B) at 2 weeks post-transplantation in both the ipsi- and contralesional cortex compared with vehicle-treated animals (n = 4–5 per group). These effects are sustained to 4 weeks post-stroke (n = 5 per group) only in the ipsilesional hemisphere. (C) Representative image of a golgi-stained layer V neuron. (D) Schematic illustrating how branch order is counted for apical and basilar dendrites. (E and F) At 4 weeks post-transplantation human NPCs enhance branching in the middle order branches in ipsilesional layer V neurons. This is more significant in basilar branches (E) compared with apical branches (F). *P < 0.05; **P < 0.01; ***P < 0.001; #P = 0.05.
Figure 3
Human NPCs enhance axonal sprouting post-stroke. (A) Representative confocal images of biotinylated dextran amine (BDA) staining in the contra- and ipsilateral corpus callosum of human NPC- and vehicle-treated animals at 5 weeks post-transplantation. Scale bar = 100 µm. (B) Human NPC-grafted rats have significantly increased BDA-labelled fibre density at 3 weeks (n = 6) and 5 weeks (n = 12) post-transplantation in the cortex, corpus callosum and the ipsilesional striatum. At 5 weeks post-transplantation human NPC-grafted rats also have increased BDA signal in (C) the ipsi- and contralesional thalamus and (D) corticospinal fibres in the contralesional internal capsule and both the contra- and ipsilesional dorsal funiculus in the cervical spinal cord, C5 level. *P < 0.05 human NPC compared with vehicle at same time point. Red oval = BDA injection site; black oval = human NPC transplant site.
Figure 4
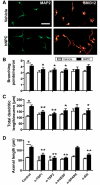
Identification of secreted factors mediating neurite plasticity in vitro. (A) Representative images of cortical neurons stained for microtubule-associated protein 2 (MAP2, labels dendrites) and SMI312 (labels axons) co-cultured without (vehicle) and with human NPCs. Scale = 50 µm. (B–D) Human NPCs significantly promoted dendritic branching (B), total dendritic length (C) and axonal outgrowth (D) of the cortical neurons; *P < 0.05 ‘control human NPC’ compared with ‘control vehicle’. Neutralization of the secreted factors with antibodies or the soluble slit receptor Roundabout-Fc significantly reduced the effects of human NPCs on these parameters as indicated. *P < 0.05, **P < 0.01 compared with ‘control human NPC’; #P < 0.05 compared with ‘control vehicle’. TSP = thrombospondins.
Figure 5
Effects of human NPCs on anterograde axonal transport in vivo and in vitro. (A) Representative confocal images of amyloid precursor protein (APP) and SMI312 staining of the corpus callosum at 6 weeks post-stroke (5 weeks post-transplant) in uninjured (naïve), vehicle treated (dMCAo, vehicle) and human NPC-grafted (dMCAo, hNPC) animals. Scale = 20 µm (B) Quantification of the percentage of amyloid precursor protein-positive SMI312 puncta to determine the percentage of amyloid precursor protein-positive axons; 3 weeks, n = 6; 5 weeks n = 12. (C) Quantification of total axonal density at 6 weeks post-stroke (n = 12). (D) Schematic of microfluidic assay system for analysis of axonal transport in vitro. (E) Time-lapse confocal images of dextran-labelled vesicles used to measure the rate of axonal transport. Arrowhead indicates the same vesicle at different times. Scale = 5 µm. (F) Human NPCs enhance the rate of axonal transport in cortical neurons compared with vehicle treatment. Neutralization of VEGF, but not other factors significantly reduced this human NPC-mediated effect. *P < 0.05, **P < 0.01. dMCAo = distal middle cerebral artery occlusion.
Comment in
- Therapeutic stem cell plasticity orchestrates tissue plasticity.
Martino G, Bacigaluppi M, Peruzzotti-Jametti L. Martino G, et al. Brain. 2011 Jun;134(Pt 6):1585-7. doi: 10.1093/brain/awr115. Brain. 2011. PMID: 21616966 No abstract available.
References
- Andres RH, Ducray AD, Huber AW, Perez-Bouza A, Krebs SH, Schlattner U, et al. Effects of creatine treatment on survival and differentiation of GABA-ergic neurons in cultured striatal tissue. J Neurochem. 2005;95:33–45. - PubMed
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. - PubMed
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. - PubMed
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS058784/NS/NINDS NIH HHS/United States
- R01NS058784/NS/NINDS NIH HHS/United States
- P01NS057778/NS/NINDS NIH HHS/United States
- P01NS37520/NS/NINDS NIH HHS/United States
- R01NS27292/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources