Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance - PubMed (original) (raw)
Randomized Controlled Trial
. 2011 Jun;60(6):1647-56.
doi: 10.2337/db10-1791.
Giuseppe Matarese, Aoife M Brennan, John P Chamberland, Xiaowen Liu, Christina G Fiorenza, Geetha H Mylvaganam, Luisa Abanni, Fortunata Carbone, Catherine J Williams, Alex M De Paoli, Benjamin E Schneider, Christos S Mantzoros
Affiliations
- PMID: 21617185
- PMCID: PMC3114380
- DOI: 10.2337/db10-1791
Randomized Controlled Trial
Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance
Hyun-Seuk Moon et al. Diabetes. 2011 Jun.
Abstract
Objective: Metreleptin has been efficacious in improving metabolic control in patients with lipodystrophy, but its efficacy has not been tested in obese patients with type 2 diabetes.
Research design and methods: We studied the role of leptin in regulating the endocrine adaptation to long-term caloric deprivation and weight loss in obese diabetic subjects over 16 weeks in the context of a double-blinded, placebo-controlled, randomized trial. We then performed detailed interventional and mechanistic signaling studies in humans in vivo, ex vivo, and in vitro.
Results: In obese patients with diabetes, metreleptin administration for 16 weeks did not alter body weight or circulating inflammatory markers but reduced HbA(1c) marginally (8.01 ± 0.93-7.96 ± 1.12, P = 0.03). Total leptin, leptin-binding protein, and antileptin antibody levels increased, limiting free leptin availability and resulting in circulating free leptin levels of ∼50 ng/mL. Consistent with clinical observations, all metreleptin signaling pathways studied in human adipose tissue and peripheral blood mononuclear cells were saturable at ∼50 ng/mL, with no major differences in timing or magnitude of leptin-activated STAT3 phosphorylation in tissues from male versus female or obese versus lean humans in vivo, ex vivo, or in vitro. We also observed for the first time that endoplasmic reticulum (ER) stress in human primary adipocytes inhibits leptin signaling.
Conclusions: In obese patients with diabetes, metreleptin administration did not alter body weight or circulating inflammatory markers but reduced HbA(1c) marginally. ER stress and the saturable nature of leptin signaling pathways play a key role in the development of leptin tolerance in obese patients with diabetes.
Figures
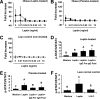
FIG. 1.
Laboratory study I. Agonistic/stimulatory activity of antileptin antibodies generated during metreleptin administration. A–C: The functional activity of antileptin antibodies in hLepR+BAF3 cells was as described in detail in
research design and methods
. ■, Leptin + IgG posttreatment; ♦, Leptin + IgG pretreatment. D–F: The biochemical level of the capacity of antileptin IgGs in hLepR+BAF3 cells was studied as described in detail in
research design and methods
. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 6) ± SD. Means with different letters are significantly different, P < 0.05. L.N.C., lean normal control.
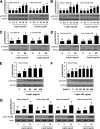
FIG. 2.
Laboratory study II. Comparative evaluation of ex vivo metreleptin signaling in hAT and hPBMCs from lean and obese subjects. Ex vivo metreleptin administration in hAT and hPBMCs was performed as described in detail in
research design and methods
. hAT (A–D) and hPBMCs (E) were incubated and stimulated with or without ex vivo metreleptin at the indicated concentrations for 30 min. F: hPBMCs were incubated and stimulated with or without ex vivo metreleptin at the indicated times. G: hAT was incubated and stimulated with or without ex vivo metreleptin at the indicated concentrations for 30 min. All lysates were examined by Western blotting as described in detail in
research design and methods
. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 3) ± SD. Means with different letters are significantly different, P < 0.05. OM, omental; SC, subcutaneous.
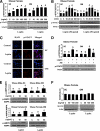
FIG. 3.
Laboratory study III. In vitro metreleptin signaling in subcutaneous (SC) and omental (OM) hPA from lean and obese subjects. In vitro metreleptin administration in hPA was performed as described in detail in
research design and methods
. A: Cells were treated with metreleptin at the indicated concentrations for 30 min. B: Cells were treated with metreleptin at the indicated times. C: Cells were treated with metreleptin (50 ng/mL) for 30 min. Immunodetection was carried out as described in detail in
research design and methods
. All pictures were ×40 magnification. D: Cells were pretreated with the STAT3 inhibitor AG490 (AG, 1 μmol/L) for 1 h, followed by treatment with 50 ng/mL metreleptin for 30 min. E and F: Cells were treated with metreleptin at the indicated concentrations for 30 min. All lysates were examined by Western blotting as described in detail in
research design and methods
. All density values for each protein band of interest are expressed as a fold increase. Data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means (n = 3) ± SD. Means with different letters are significantly different, P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
Similar articles
- Metreleptin: first global approval.
Chou K, Perry CM. Chou K, et al. Drugs. 2013 Jun;73(9):989-97. doi: 10.1007/s40265-013-0074-7. Drugs. 2013. PMID: 23740412 Review. - Identification and saturable nature of signaling pathways induced by metreleptin in humans: comparative evaluation of in vivo, ex vivo, and in vitro administration.
Moon HS, Huh JY, Dincer F, Schneider BE, Hasselgren PO, Mantzoros CS. Moon HS, et al. Diabetes. 2015 Mar;64(3):828-39. doi: 10.2337/db14-0625. Epub 2014 Sep 23. Diabetes. 2015. PMID: 25249580 Free PMC article. Clinical Trial. - Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy.
Chan JL, Koda J, Heilig JS, Cochran EK, Gorden P, Oral EA, Brown RJ. Chan JL, et al. Clin Endocrinol (Oxf). 2016 Jul;85(1):137-49. doi: 10.1111/cen.12980. Epub 2016 Feb 2. Clin Endocrinol (Oxf). 2016. PMID: 26589105 Free PMC article. Clinical Trial. - Intracellular leptin signaling following effective weight loss.
Sahin-Efe A, Polyzos SA, Dincer F, Zaichenko L, McGovern R, Schneider B, Mantzoros CS. Sahin-Efe A, et al. Metabolism. 2015 Aug;64(8):888-95. doi: 10.1016/j.metabol.2015.04.006. Epub 2015 May 1. Metabolism. 2015. PMID: 25998856 - Metreleptin and generalized lipodystrophy and evolving therapeutic perspectives.
Tchang BG, Shukla AP, Aronne LJ. Tchang BG, et al. Expert Opin Biol Ther. 2015 Jul;15(7):1061-75. doi: 10.1517/14712598.2015.1052789. Expert Opin Biol Ther. 2015. PMID: 26063386 Review.
Cited by
- Differential associations of leptin with adiposity across early childhood.
Boeke CE, Mantzoros CS, Hughes MD, L Rifas-Shiman S, Villamor E, Zera CA, Gillman MW. Boeke CE, et al. Obesity (Silver Spring). 2013 Jul;21(7):1430-7. doi: 10.1002/oby.20314. Epub 2013 May 25. Obesity (Silver Spring). 2013. PMID: 23408391 Free PMC article. - Metreleptin: first global approval.
Chou K, Perry CM. Chou K, et al. Drugs. 2013 Jun;73(9):989-97. doi: 10.1007/s40265-013-0074-7. Drugs. 2013. PMID: 23740412 Review. - The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice.
List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. List EO, et al. Mol Endocrinol. 2013 Mar;27(3):524-35. doi: 10.1210/me.2012-1330. Epub 2013 Jan 24. Mol Endocrinol. 2013. PMID: 23349524 Free PMC article. - Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals.
Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, Paruthi J, Mantzoros CS. Moon HS, et al. Endocr Rev. 2013 Jun;34(3):377-412. doi: 10.1210/er.2012-1053. Epub 2013 Mar 8. Endocr Rev. 2013. PMID: 23475416 Free PMC article. Review. - Inflammation Markers in Type 2 Diabetes and the Metabolic Syndrome in the Pediatric Population.
Reinehr T, Roth CL. Reinehr T, et al. Curr Diab Rep. 2018 Oct 18;18(12):131. doi: 10.1007/s11892-018-1110-5. Curr Diab Rep. 2018. PMID: 30338401 Review.
References
- Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–578 - PubMed
- Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab 2005;90:1618–1624 - PubMed
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–529 - PubMed
- Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 - PubMed
- Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cell apoptosis. Endocrinology 2006;147:3398–3407 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 DK081913/DK/NIDDK NIH HHS/United States
- 202579/ERC_/European Research Council/International
- M01 RR001032/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- F32-DK64550-01A1/DK/NIDDK NIH HHS/United States
- R01 DK058785/DK/NIDDK NIH HHS/United States
- R56 DK058785/DK/NIDDK NIH HHS/United States
- GJT08004/TI_/Telethon/Italy
- DK58785/DK/NIDDK NIH HHS/United States
- AG032030/AG/NIA NIH HHS/United States
- DK79929/DK/NIDDK NIH HHS/United States
- R01 AG032030/AG/NIA NIH HHS/United States
- R01 DK079929/DK/NIDDK NIH HHS/United States
- DK081913/DK/NIDDK NIH HHS/United States
- F32 DK064550/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous