Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature - PubMed (original) (raw)
. 2011 Jun 10;88(6):788-795.
doi: 10.1016/j.ajhg.2011.04.019. Epub 2011 May 27.
Orianne Philippe 2, Annick Raas-Rothschild 3, Sebastian H Eck 4, Elisabeth Graf 4, Rebecca Buchert 5, Guntram Borck 2, Arif Ekici 5, Felix F Brockschmidt 6, Markus M Nöthen 6, Arnold Munnich 2, Tim M Strom 7, Andre Reis 5, Laurence Colleaux 8
Affiliations
- PMID: 21620353
- PMCID: PMC3113253
- DOI: 10.1016/j.ajhg.2011.04.019
Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature
Rami Abou Jamra et al. Am J Hum Genet. 2011.
Abstract
Intellectual disability inherited in an autosomal-recessive fashion represents an important fraction of severe cognitive-dysfunction disorders. Yet, the extreme heterogeneity of these conditions markedly hampers gene identification. Here, we report on eight affected individuals who were from three consanguineous families and presented with severe intellectual disability, absent speech, shy character, stereotypic laughter, muscular hypotonia that progressed to spastic paraplegia, microcephaly, foot deformity, decreased muscle mass of the lower limbs, inability to walk, and growth retardation. Using a combination of autozygosity mapping and either Sanger sequencing of candidate genes or next-generation exome sequencing, we identified one mutation in each of three genes encoding adaptor protein complex 4 (AP4) subunits: a nonsense mutation in AP4S1 (NM_007077.3: c.124C>T, p.Arg42(∗)), a frameshift mutation in AP4B1 (NM_006594.2: c.487_488insTAT, p.Glu163_Ser739delinsVal), and a splice mutation in AP4E1 (NM_007347.3: c.542+1_542+4delGTAA, r.421_542del, p.Glu181Glyfs(∗)20). Adaptor protein complexes (AP1-4) are ubiquitously expressed, evolutionarily conserved heterotetrameric complexes that mediate different types of vesicle formation and the selection of cargo molecules for inclusion into these vesicles. Interestingly, two mutations affecting AP4M1 and AP4E1 have recently been found to cause cerebral palsy associated with severe intellectual disability. Combined with previous observations, these results support the hypothesis that AP4-complex-mediated trafficking plays a crucial role in brain development and functioning and demonstrate the existence of a clinically recognizable syndrome due to deficiency of the AP4 complex.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
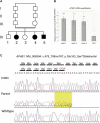
Figure 1
Genetic Analysis of Family ID01 (A) Pedigree of the family. (B) Electrophoregrams illustrating the c.487_488insTAT, p.Glu163_Ser739delinsVal variant in exon 5 of AP4B1. Data are shown for homozygous affected individuals, heterozygous healthy parents, and homozygous wild-type healthy siblings. (C) Quantitative RT-PCR analysis of AP4B1 mRNA. AP4B1 expression in fibroblast cells from three controls and from patient IV-2. Data are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Means ± standard deviation are given (n = 3 independent experiments). ∗∗∗p value of < 0.01 (Student's test) for the difference of expression. ∗∗p value of < 0.05 (Student's test) for the difference of expression.
Figure 2
Genetic Analysis of Family MR061 (A) Pedigree of family; arrows indicate index. Family MR061 is large with multiple affected individuals with variable phenotypes. Grey symbols denote individuals in whom clinical presentation is markedly different and who have no AP4S1 mutation (heterogeneity within the family). (B) Electropherograms illustrating the mutation in exon 2 of AP4S1. (C) Facial appearance of affected individuals with discreet remarkable facial gestalt, including a prominent and bulbous nose, a wide mouth, and coarse features and photographs of lower limbs with foot deformity and decreased muscle mass of the shanks.
Figure 3
Genetic Analysis of Family MR071 (A) Pedigree of family MR071. (B) Representative sequence traces from cDNA showing skipping of exon 5. (C) Facial appearance of affected individuals includes discreet remarkable facial gestalt with prominent and bulbous nose, wide mouth, and coarse features. Also shown are photographs of the lower limbs with foot deformity and decreased muscle mass of the shanks. (D) RT-PCR products of mRNA from homozygous affected individuals, heterozygous healthy parents, and homozygous wild-type healthy siblings; the expected size from the normal AP4E1 allele (512 bp) as well as a smaller band corresponding to aberrant splicing of the mutated allele with skipping of exon 5 (389 bp) is shown.
Similar articles
- An AP4B1 frameshift mutation in siblings with intellectual disability and spastic tetraplegia further delineates the AP-4 deficiency syndrome.
Abdollahpour H, Alawi M, Kortüm F, Beckstette M, Seemanova E, Komárek V, Rosenberger G, Kutsche K. Abdollahpour H, et al. Eur J Hum Genet. 2015 Feb;23(2):256-9. doi: 10.1038/ejhg.2014.73. Epub 2014 Apr 30. Eur J Hum Genet. 2015. PMID: 24781758 Free PMC article. - Autosomal recessive spastic tetraplegia caused by AP4M1 and AP4B1 gene mutation: expansion of the facial and neuroimaging features.
Tüysüz B, Bilguvar K, Koçer N, Yalçınkaya C, Çağlayan O, Gül E, Sahin S, Çomu S, Günel M. Tüysüz B, et al. Am J Med Genet A. 2014 Jul;164A(7):1677-85. doi: 10.1002/ajmg.a.36514. Epub 2014 Apr 3. Am J Med Genet A. 2014. PMID: 24700674 - A novel homozygous AP4B1 mutation in two brothers with AP-4 deficiency syndrome and ocular anomalies.
Accogli A, Hamdan FF, Poulin C, Nassif C, Rouleau GA, Michaud JL, Srour M. Accogli A, et al. Am J Med Genet A. 2018 Apr;176(4):985-991. doi: 10.1002/ajmg.a.38628. Epub 2018 Feb 12. Am J Med Genet A. 2018. PMID: 29430868 - An Ultra-Rare Mixed Phenotype with Combined AP-4 and ERF Mutations: The First Report in a Pediatric Patient and a Literature Review.
Orsini A, Santangelo A, Carmignani A, Camporeale A, Massart F, Tyutyusheva N, Peroni DG, Foiadelli T, Ferretti A, Toschi B, Romano S, Bonuccelli A. Orsini A, et al. Genes (Basel). 2024 Mar 29;15(4):436. doi: 10.3390/genes15040436. Genes (Basel). 2024. PMID: 38674371 Free PMC article. Review. - Adaptor protein complexes AP-4 and AP-5: new players in endosomal trafficking and progressive spastic paraplegia.
Hirst J, Irving C, Borner GH. Hirst J, et al. Traffic. 2013 Feb;14(2):153-64. doi: 10.1111/tra.12028. Epub 2012 Dec 7. Traffic. 2013. PMID: 23167973 Review.
Cited by
- The History of Gene Hunting in Hereditary Spinocerebellar Degeneration: Lessons From the Past and Future Perspectives.
Yahia A, Stevanin G. Yahia A, et al. Front Genet. 2021 Mar 23;12:638730. doi: 10.3389/fgene.2021.638730. eCollection 2021. Front Genet. 2021. PMID: 33833777 Free PMC article. Review. - DYNC2LI1 mutations broaden the clinical spectrum of dynein-2 defects.
Kessler K, Wunderlich I, Uebe S, Falk NS, Gießl A, Brandstätter JH, Popp B, Klinger P, Ekici AB, Sticht H, Dörr HG, Reis A, Roepman R, Seemanová E, Thiel CT. Kessler K, et al. Sci Rep. 2015 Jul 1;5:11649. doi: 10.1038/srep11649. Sci Rep. 2015. PMID: 26130459 Free PMC article. - Golgipathies reveal the critical role of the sorting machinery in brain and skeletal development.
El Ghouzzi V, Boncompain G. El Ghouzzi V, et al. Nat Commun. 2022 Dec 1;13(1):7397. doi: 10.1038/s41467-022-35101-y. Nat Commun. 2022. PMID: 36456556 Free PMC article. - Recessive Mutations in AP1B1 Cause Ichthyosis, Deafness, and Photophobia.
Boyden LM, Atzmony L, Hamilton C, Zhou J, Lim YH, Hu R, Pappas J, Rabin R, Ekstien J, Hirsch Y, Prendiville J, Lifton RP, Ferguson S, Choate KA. Boyden LM, et al. Am J Hum Genet. 2019 Nov 7;105(5):1023-1029. doi: 10.1016/j.ajhg.2019.09.021. Epub 2019 Oct 17. Am J Hum Genet. 2019. PMID: 31630788 Free PMC article. - Locus and allelic heterogeneity in five families with hereditary spastic paraplegia.
Hebbar M, Shukla A, Nampoothiri S, Bielas S, Girisha KM. Hebbar M, et al. J Hum Genet. 2019 Jan;64(1):17-21. doi: 10.1038/s10038-018-0523-y. Epub 2018 Oct 18. J Hum Genet. 2019. PMID: 30337681 Free PMC article.
References
- Ropers H.H. Genetics of early onset cognitive impairment. Annu. Rev. Genomics Hum. Genet. 2010;11:161–187. - PubMed
- Rauch A., Hoyer J., Guth S., Zweier C., Kraus C., Becker C., Zenker M., Hüffmeier U., Thiel C., Rüschendorf F. Diagnostic yield of various genetic approaches in patients with unexplained developmental delay or mental retardation. Am. J. Med. Genet. A. 2006;140:2063–2074. - PubMed
- Ropers H.H., Hamel B.C. X-linked mental retardation. Nat. Rev. Genet. 2005;6:46–57. - PubMed
- Çalışkan M., Chong J.X., Uricchio L., Anderson R., Chen P., Sougnez C., Garimella K., Gabriel S.B., dePristo M.A., Shakir K. Exome sequencing reveals a novel mutation for autosomal recessive non-syndromic mental retardation in the TECR gene on chromosome 19p13. Hum. Mol. Genet. 2011;20:1285–1289. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases