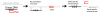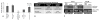Polymer delivery systems for site-specific genome editing - PubMed (original) (raw)
Review
Polymer delivery systems for site-specific genome editing
Nicole Ali McNeer et al. J Control Release. 2011.
Abstract
Triplex-forming peptide nucleic acids (PNAs) can be used to coordinate the recombination of short 50-60bp "donor DNA" fragments into genomic DNA, resulting in site-specific correction of genetic mutations or the introduction of advantageous genetic modifications. Site-specific gene editing in hematopoietic stem and progenitor cells (HSPCs) could result in the treatment or cure of inherited disorders of the blood such as β-thalassemia or sickle cell anemia. Gene editing in HSPCs and differentiated T cells could also help combat HIV infection by modifying the HIV co-receptor CCR5, which is necessary for R5-tropic HIV entry. However, translation of genome modification technologies to clinical practice is limited by challenges in intracellular delivery, especially in difficult-to-transfect hematolymphoid cells. Here, we review the use of engineered biodegradable polymer nanoparticles for site-specific genome editing in human hematopoietic cells, which represent a promising approach for ex vivo and in vivo gene therapy.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
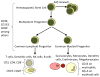
Figure 1. Hematopoeisis
A schematic of hematopoiesis, the process by which hematopoietic stem cells form the components of the blood and immune system. Some examples of cell surface markers are included.
Figure 2. Genome modification using PNA and DNA
The 50 to 60-mer donor DNA (black) is homologous to the gene target of choice (grey) except for a several base-pair mutation (X X X). The PNA (red) binds near the target and induces homologous recombination of the donor strand into the target. Allele-specific PCR (AS-PCR) can distinguish between modified (mutant) and unmodified (wild-type) genomic DNA.
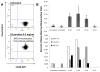
Figure 3. Triplex-forming PNAs induce recombination at a thalassemia mutation site
(A) PNAs were designed to bind to intron 2 sequences of the human b-globin gene at a distance of 35 to 830 bp from the targeted thalassemia mutation at the first position of intron 2. (B) PNA-mediated gene correction frequencies in CHO cells using a GFP-expression assay. (C) Human CD34+ cells treated with PNAs and short donor DNAs (HBB donor) were capable of differentiation into erythroid and neutrophil lineages; both lineages showed targeted gene modification by AS-PCR up to 21 days following oligonucleotide transfection. Reproduced with permission from [9] (permission pending).
Figure 4. Schematic of nanoparticle formulation
PNA and DNA were loaded into nanoparticles using a previously described double-emulsion solvent evaporation technique [29]. In combined PNA-DNA particles, PNA acts as the counterion for DNA. In DNA only particles, spermidine is added to act as the counterion. Scanning electron micrograph shows sample batch of PNA-DNA nanoparticles.
Figure 5. PLGA nanoparticles deliver cargo into hematopoietic cells in vitro and in vivo
(A) Human CD34+ cells were treated with 0.2 mg/mL of C6 PLGA particles at a cell concentration of 1 million cells/mL. Cells were harvested for FACS for analysis 24 hours post-treatment, and either co-stained with CD34 APC (an HSPC marker) or trypan blue (quenches external fluorescence). Co-staining with trypan blue indicated that 39% of fluorescence was from internalized particles. (B) 17 week old NCr nu/nu mice were injected with either 9.6 mg IP or 6 mg IV PLGA nanoparticles containing C6, in RPMI media (320 μL or 200 μL respectively). Mice were then sacrificed at 6 hours to assess for nanoparticle uptake in bone marrow cells by FACS. Total percentages of C6 positive cells or percentages co-staining for C6 and specific cell markers are given.
Fig. 6. PNA-DNA nanoparticles mediate genomic modification in the β-globin gene in HSPCs with low toxicity and high efficiency
Blank particles contain PBS. For nucleofection, cells were nucleofected as per the Amaxa protocol. Mock nucleofection was without nucleic acid. (A) Nucleic acid delivery by nanoparticles has higher live cell recovery than nucleofection. Cell counts were performed after 3 days of treatment using Trypan blue to stain dead cells. Error bars give SD. *** p = 5×10^-12 for two-tailed t-test, 2-sample unequal variance. (B) PNA-DNA delivery by nanoparticles leads to higher gene modification frequencies than nucleofection. Cells were treated with 2 mg/mL nanoparticles or optimized Amaxa nucleofection. Modification frequencies were determined by two independent methods after three days of treatment, mean and 95% confidence intervals given. Standard curve qPCR: Quantitative AS-PCR was performed on genomic DNA from treated cells, and relative values were compared to a standard curve generated by known amounts of mutant plasmid copies in wild-type genomic DNA. Limiting dilution: after the three day treatment, cells were replated at 20 cells/well in 96-well format and expanded 4 weeks in replica plates. Genomic DNA was harvested and assessed by AS-PCR. Positive wells were validated in the replica. Limiting dilution analysis (
http://bioinf.wehi.edu.au/software/elda
) was used to determine frequencies. (C) Cells maintain mutation after differentiation and expansion. CD34+ cells were treated with 0.5 mg/mL particles and then switched to erythroid- or neutrophil-differentiating conditions, or in media with expansion (non-differentiating) cytokines. Reproduced with permission from [23].
Similar articles
- Nanoparticles deliver triplex-forming PNAs for site-specific genomic recombination in CD34+ human hematopoietic progenitors.
McNeer NA, Chin JY, Schleifman EB, Fields RJ, Glazer PM, Saltzman WM. McNeer NA, et al. Mol Ther. 2011 Jan;19(1):172-80. doi: 10.1038/mt.2010.200. Epub 2010 Sep 21. Mol Ther. 2011. PMID: 20859257 Free PMC article. - Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo.
McNeer NA, Schleifman EB, Cuthbert A, Brehm M, Jackson A, Cheng C, Anandalingam K, Kumar P, Shultz LD, Greiner DL, Mark Saltzman W, Glazer PM. McNeer NA, et al. Gene Ther. 2013 Jun;20(6):658-69. doi: 10.1038/gt.2012.82. Epub 2012 Oct 18. Gene Ther. 2013. PMID: 23076379 Free PMC article. - Single-stranded γPNAs for in vivo site-specific genome editing via Watson-Crick recognition.
Bahal R, Quijano E, McNeer NA, Liu Y, Bhunia DC, Lopez-Giraldez F, Fields RJ, Saltzman WM, Ly DH, Glazer PM. Bahal R, et al. Curr Gene Ther. 2014;14(5):331-42. doi: 10.2174/1566523214666140825154158. Curr Gene Ther. 2014. PMID: 25174576 Free PMC article. - Peptide Nucleic Acids and Gene Editing: Perspectives on Structure and Repair.
Economos NG, Oyaghire S, Quijano E, Ricciardi AS, Saltzman WM, Glazer PM. Economos NG, et al. Molecules. 2020 Feb 8;25(3):735. doi: 10.3390/molecules25030735. Molecules. 2020. PMID: 32046275 Free PMC article. Review. - Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing.
Ricciardi AS, Quijano E, Putman R, Saltzman WM, Glazer PM. Ricciardi AS, et al. Molecules. 2018 Mar 11;23(3):632. doi: 10.3390/molecules23030632. Molecules. 2018. PMID: 29534473 Free PMC article. Review.
Cited by
- Therapeutic Peptide Nucleic Acids: Principles, Limitations, and Opportunities.
Quijano E, Bahal R, Ricciardi A, Saltzman WM, Glazer PM. Quijano E, et al. Yale J Biol Med. 2017 Dec 19;90(4):583-598. eCollection 2017 Dec. Yale J Biol Med. 2017. PMID: 29259523 Free PMC article. Review. - Therapeutic genome mutagenesis using synthetic donor DNA and triplex-forming molecules.
Reza F, Glazer PM. Reza F, et al. Methods Mol Biol. 2015;1239:39-73. doi: 10.1007/978-1-4939-1862-1_4. Methods Mol Biol. 2015. PMID: 25408401 Free PMC article. - Using Gene Editing Approaches to Fine-Tune the Immune System.
Pavlovic K, Tristán-Manzano M, Maldonado-Pérez N, Cortijo-Gutierrez M, Sánchez-Hernández S, Justicia-Lirio P, Carmona MD, Herrera C, Martin F, Benabdellah K. Pavlovic K, et al. Front Immunol. 2020 Sep 29;11:570672. doi: 10.3389/fimmu.2020.570672. eCollection 2020. Front Immunol. 2020. PMID: 33117361 Free PMC article. Review. - The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders.
Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. Borlongan CV, et al. Prog Neurobiol. 2011 Oct;95(2):213-28. doi: 10.1016/j.pneurobio.2011.08.005. Epub 2011 Aug 30. Prog Neurobiol. 2011. PMID: 21903148 Free PMC article. Review. - Gene-targeting pharmaceuticals for single-gene disorders.
Beaudet AL, Meng L. Beaudet AL, et al. Hum Mol Genet. 2016 Apr 15;25(R1):R18-26. doi: 10.1093/hmg/ddv476. Epub 2015 Nov 30. Hum Mol Genet. 2016. PMID: 26628634 Free PMC article. Review.
References
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382(6593):722–725. - PubMed
- Papapetrou EP, Zoumbos NC, Athanassiadou A. Genetic modification of hematopoietic stem cells with nonviral systems: past progress and future prospects. Gene Ther. 2005;12(Suppl 1):S118–130. - PubMed
- Persons DA. The challenge of obtaining therapeutic levels of genetically modified hematopoietic stem cells in beta-thalassemia patients. Ann N Y Acad Sci. 2010;1202:69–74. - PubMed
- Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32GM07205/GM/NIGMS NIH HHS/United States
- F30 HL110372/HL/NHLBI NIH HHS/United States
- HL085416/HL/NHLBI NIH HHS/United States
- R01 EB000487/EB/NIBIB NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- R56 EB000487/EB/NIBIB NIH HHS/United States
- R01 HL085416/HL/NHLBI NIH HHS/United States
- R01 HL082655/HL/NHLBI NIH HHS/United States
- HL082655/HL/NHLBI NIH HHS/United States
- EB000487/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources