Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability - PubMed (original) (raw)
Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability
M Rizwan Siddiqui et al. J Cell Biol. 2011.
Abstract
Endothelial barrier function is regulated by adherens junctions (AJs) and caveolae-mediated transcellular pathways. The opening of AJs that is observed in caveolin-1(-/-) (Cav-1(-/-)) endothelium suggests that Cav-1 is necessary for AJ assembly or maintenance. Here, using endothelial cells isolated from Cav-1(-/-) mice, we show that Cav-1 deficiency induced the activation of endothelial nitric oxide synthase (eNOS) and the generation of nitric oxide (NO) and peroxynitrite. We assessed S-nitrosylation and nitration of AJ-associated proteins to identify downstream NO redox signaling targets. We found that the GTPase-activating protein (GAP) p190RhoGAP-A was selectively nitrated at Tyr1105, resulting in impaired GAP activity and RhoA activation. Inhibition of eNOS or RhoA restored AJ integrity and diminished endothelial hyperpermeability in Cav-1(-/-) mice. Thrombin, a mediator of increased endothelial permeability, also induced nitration of p120-catenin-associated p190RhoGAP-A. Thus, eNOS-dependent nitration of p190RhoGAP-A represents a crucial mechanism for AJ disassembly and resultant increased endothelial permeability.
Figures
Figure 1.
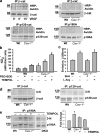
Cav-1 deficiency induces eNOS activation and AJ destabilization in endothelium. (a) Phosphorylation of eNOS at S1177 and Akt-1 at S473 in Wt and _Cav-1_−/− MLVECs; n = 3. Molecular mass standards are indicated next to the gel blots in kilodaltons. (b) Basal NO generation was measured in the absence or presence of
l
-NNA or NPA and 1400W in combination. Arrow,
l
-arginine replenishment. Scale bars are shown for 100 nM NO and 10 min. Bar plot, NO accumulation in a media over 20 min; mean ± SEM (error bars); *, P < 0.05 as compared with Wt control; n = 6. (c) Wt and _Cav-1_−/− endothelial monolayers stained for VE-cadherin, β-catenin (green in overlays), F-actin (red), and nuclei (blue). Inter-endothelial gaps in _Cav-1_−/− monolayers are indicated by arrowheads. Bar, 10 µm. (d and e) Accumulation of β-catenin at AJs was expressed as a mean pixel intensity of threshold area shown in d (threshold above intracellular background is in orange). (f) Area of inter-endothelial gaps was determined using the same set of images as in e; n = 17. Black circles and error bars in e and f indicate mean and SEM, respectively; *, P < 0.01 as compared with Wt control.
Figure 2.
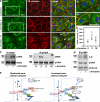
Peroxynitrite generation in _Cav-1_−/− endothelium and nitration of p190A. (a) S-nitrosylation of β- and p120-catenins, and p190A. Proteins were co-immunoprecipitated with specific Abs, and precipitates were probed with HRP-avidin; results of two independent experiments are shown; n = 4. Note that the β-catenin band is indicated by an arrow. SNO of β-catenin in VEGF-treated cells is a positive control. Molecular mass standards are indicated next to the gel blots in kilodaltons. (b) The bar plot shows nitrite accumulation in the presence of SOD and TEMPOL; mean ± SEM (error bars); *, P < 0.05 as compared with Wt control; n = 4. (c) Bar plot shows the fluorescence emission of EOH at 580 nm; mean ± SEM (error bars); *, P < 0.05 as compared with Wt control; n = 4. (d) β- and p120-catenins and (e) p190A were co-immunoprecipitated with specific Abs, and precipitates were probed for nitrotyrosine (3-N). Results of two independent experiments are shown; n = 4. (e) Cav-1 deletion induced the nitration of p190A, which was reduced by TEMPOL treatment and in cells isolated from lungs of Cav-1/eNOS double knockout mice (DKO). (e, right) Bar plot; fold increase in p190A nitration normalized to p190A loading; mean ± SEM (error bars); *, P < 0.05 as compared with Wt; n = 4.
Figure 3.
Nitration of p190A induces RhoA activation in endothelial cells. (a) p190A was co-immunoprecipitated and precipitates were probed for phosphotyrosine (PY20) and p190A; n = 3. Molecular mass standards are indicated next to the gel blots in kilodaltons. (b) Human microvascular endothelial cells (dermal) overexpressing HA-tagged p190A, p190B, or p190A mutants Y1105F and Y1087F were treated with SIN-1. Nitration of endogenous p190A and exogenously expressed proteins was determined with 3-N antibody. Exogenous proteins were co-immunoprecipitated with anti-HA antibody; n = 3. (c) Live imaging of a genetically encoded FRET-based RhoA biosensor. Representative YFP and FRET/CFP ratio confocal images. Pixel intensities of ratio images were scaled from 0 to 5 and color-coded as indicated on the left. Bar, 10 µm. (d) FRET/CFP emission ratio; mean and SEM are as in Fig. 1 e; n = 13; *, P < 0.01. (e) RhoA-GTP was pulled down with Rhotekin-RBD beads. Resultant precipitates and 5% of cell extracts were probed for RhoA; results of two independent experiments are shown; n = 4. Molecular mass standards are indicated next to the gel blots in kilodaltons.
Figure 4.
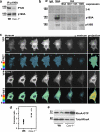
Restoration of normal paracellular permeability in _Cav-1_−/− endothelial monolayers and vessels by inhibition of either RhoA or eNOS. (a) Immunofluorescent staining of MLVECs isolated from _Cav-1_−/− and Cav-1/eNOS double knockout (DKO) mice for β-catenin (green), F-actin (red), and nuclei (blue). _Cav-1_−/− cells were treated with Rho inhibitor C3 transferase and AP-CSD peptide. Bar, 10 µm. (b) β-catenin accumulation at AJs as in Fig. 1 e; *, P < 0.01 as compared with Wt control; **, P < 0.01 as compared with _Cav-1_−/−; n = 10. (c) Endothelial permeability to EBA; mean and SEM are as in Fig. 1 e; *, P < 0.01 as compared with Wt control; **, P < 0.05 as compared with _Cav-1_−/−; n = 4. (d) Lung weight changes after a step increase in transvascular oncotic pressure gradient. Recordings were smoothed by averaging successive groups of five points. Lungs isolated from Wt and _Cav-1_−/− mice were perfused with 0% BSA for 10 min, with 10% BSA for 30 min, and with 0% BSA for 10 min; an additional _Cav-1_−/− group received AP-CSD peptide starting at 10 min of BSA profusion. AP-CSD peptide reversed lung weight loss during high albumin perfusion in _Cav-1_−/− lungs and largely restored the transvascular fluid filtration rate between 40 and 50 min. (e) The filtration rate was calculated from the initial slope of slow exponential component of lung weight gain; mean and SEM are as in Fig. 1 e; *, P < 0.05 as compared with Wt control; n = 5–9.
Figure 5.
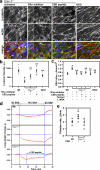
eNOS-dependent NO-redox signaling disrupts AJ integrity. (a) Immunofluorescent staining of HPAECs for eNOS (green) and β-cat (red). DAPI (blue) is shown before and after stimulation with 50 nM α-thrombin. a1 and a2 are enlarged views of boxed regions; inter-endothelial gaps are indicated by arrowheads. Bars, 10 µm. (b) eNOS accumulation at AJs was measured as in Fig. 1 e with some modification (see Materials and methods); *, P < 0.05 as compared with Wt control; n = 6. α-Thrombin induced transient accumulation of eNOS at AJs. (c and d) α-Thrombin induced transient interaction between eNOS and p190A and nitration of p190A. eNOS and p190A were co-immunoprecipitated with specific Abs from HPAECs stimulated with 50 nM α-thrombin. Precipitates were probed for p190A, eNOS, 3-N, and p120-catenin. Molecular mass standards are indicated next to the gel blots in kilodaltons. (e) Model showing that Cav-1–mediated recruitment of eNOS into caveolar domain allows formation of stable endothelial VE-cadherin adhesions, which restricts the permeability of the paracellular route to plasma proteins. Deficiency in Cav-1 or release of eNOS from caveolae results in activation of eNOS, translocation of eNOS to AJs, and transient interaction with p190A. Peroxynitrite, a product of NO and O2•−, inhibits p190A activity by nitrating Y1105. Activation of RhoA leads to reorganization of the actin cytoskeleton, actomyosin contractility, destabilization of AJs, and increased vascular permeability via the paracellular pathway.
Similar articles
- The Muscarinic Acetylcholine M2 Receptor-Induced Nitration of p190A by eNOS Increases RhoA Activity in Cardiac Myocytes.
Levay MK, Throm L, Bahrami N, Wieland T. Levay MK, et al. Cells. 2023 Oct 11;12(20):2432. doi: 10.3390/cells12202432. Cells. 2023. PMID: 37887276 Free PMC article. - The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells.
Lim EJ, Smart EJ, Toborek M, Hennig B. Lim EJ, et al. Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3340-7. doi: 10.1152/ajpheart.00921.2007. Epub 2007 Oct 12. Am J Physiol Heart Circ Physiol. 2007. PMID: 17933968 - Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis.
Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, Ghisdal P, Grégoire V, Dessy C, Balligand JL, Feron O. Sonveaux P, et al. Circ Res. 2004 Jul 23;95(2):154-61. doi: 10.1161/01.RES.0000136344.27825.72. Epub 2004 Jun 17. Circ Res. 2004. PMID: 15205364 - Nitric oxide, S-nitrosation, and endothelial permeability.
Durán WN, Beuve AV, Sánchez FA. Durán WN, et al. IUBMB Life. 2013 Oct;65(10):819-26. doi: 10.1002/iub.1204. Epub 2013 Sep 17. IUBMB Life. 2013. PMID: 24078390 Free PMC article. Review. - A novel insight into the mechanism of pulmonary hypertension involving caveolin-1 deficiency and endothelial nitric oxide synthase activation.
Zhao YY, Malik AB. Zhao YY, et al. Trends Cardiovasc Med. 2009 Oct;19(7):238-42. doi: 10.1016/j.tcm.2010.02.003. Trends Cardiovasc Med. 2009. PMID: 20382348 Free PMC article. Review.
Cited by
- VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions.
Komarova YA, Huang F, Geyer M, Daneshjou N, Garcia A, Idalino L, Kreutz B, Mehta D, Malik AB. Komarova YA, et al. Mol Cell. 2012 Dec 28;48(6):914-25. doi: 10.1016/j.molcel.2012.10.011. Epub 2012 Nov 15. Mol Cell. 2012. PMID: 23159740 Free PMC article. - Lower Serum Caveolin-1 Is Associated with Cerebral Microbleeds in Patients with Acute Ischemic Stroke.
Zhang J, Zhu W, Xiao L, Cao Q, Zhang H, Wang H, Ye Z, Hao Y, Dai Q, Sun W, Xiong Y, Liu X, Ye R, Xu G. Zhang J, et al. Oxid Med Cell Longev. 2016;2016:9026787. doi: 10.1155/2016/9026787. Epub 2016 Mar 28. Oxid Med Cell Longev. 2016. PMID: 27119011 Free PMC article. - srGAP2 deactivates RhoA to control the duration of thrombin-mediated endothelial permeability.
Lopez Rioja A, Faulkner A, Mellor H. Lopez Rioja A, et al. Vasc Biol. 2022 Feb 28;4(1):K1-K10. doi: 10.1530/VB-21-0012. eCollection 2022 Feb 1. Vasc Biol. 2022. PMID: 35441126 Free PMC article. - Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier.
Van Dyken P, Lacoste B. Van Dyken P, et al. Front Neurosci. 2018 Dec 11;12:930. doi: 10.3389/fnins.2018.00930. eCollection 2018. Front Neurosci. 2018. PMID: 30618559 Free PMC article. Review. - Regulation of Cell Signaling and Function by Endothelial Caveolins: Implications in Disease.
Sowa G. Sowa G. Transl Med (Sunnyvale). 2012;Suppl 8:001. doi: 10.4172/2161-1025.S8-001. Epub 2012 Jan 4. Transl Med (Sunnyvale). 2012. PMID: 26605130 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL103922/HL/NHLBI NIH HHS/United States
- P01 HL 60678/HL/NHLBI NIH HHS/United States
- R01 HL 45638/HL/NHLBI NIH HHS/United States
- R01 HL045638/HL/NHLBI NIH HHS/United States
- P01 HL060678/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous