Parkin mediates apparent E2-independent monoubiquitination in vitro and contains an intrinsic activity that catalyzes polyubiquitination - PubMed (original) (raw)
Parkin mediates apparent E2-independent monoubiquitination in vitro and contains an intrinsic activity that catalyzes polyubiquitination
Katherine C M Chew et al. PLoS One. 2011.
Abstract
Background: Mutations in the parkin gene, which encodes a ubiquitin ligase (E3), are a major cause of autosomal recessive parkinsonism. Although parkin-mediated ubiquitination was initially linked to protein degradation, accumulating evidence suggests that the enzyme is capable of catalyzing multiple forms of ubiquitin modifications including monoubiquitination, K48- and K63-linked polyubiquitination. In this study, we sought to understand how a single enzyme could exhibit such multifunctional catalytic properties.
Methods and findings: By means of in vitro ubiquitination assays coupled with mass spectrometry analysis, we were surprised to find that parkin is apparently capable of mediating E2-independent protein ubiquitination in vitro, an unprecedented activity exhibited by an E3 member. Interestingly, whereas full length parkin catalyzes solely monoubiquitination regardless of the presence or absence of E2, a truncated parkin mutant containing only the catalytic moiety supports both E2-independent and E2-dependent assembly of ubiquitin chains.
Conclusions: Our results here suggest a complex regulation of parkin's activity and may help to explain how a single enzyme like parkin could mediate diverse forms of ubiquitination.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Parkin mediates self ubiquitination in the presence or absence of E2.
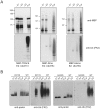
(A) In vitro ubiquitination reaction products generated by MBP-parkin, C441R or IBR-R2 in the absence of E1, E2 or parkin (i.e. “-E3”), or in the presence of all three components (Full) were subjected to immunoblotting with anti-parkin, anti-FK1 and anti-FK2, as indicated. Notice the ladders of immunoreactivities observed in MBP-parkin and IBR-R2-catalyzed reactions but not in C441R-containing reactions. (B) Reaction products generated by His-tagged parkin (His-parkin) purified from insect cells in the presence or absence of UbcH7 at various time points were subjected to immunoblotting with anti-parkin, anti-FK2 and anti-E1, as indicated. Arrows indicate His-parkin. (C) Reaction products generated by MBP-parkin or IBR-R2 in the absence of E2 at various time points were subjected to immunoblotting with anti-parkin, anti-FK1 and anti-FK2. Control refers to full reactions. (D & E) MBP-parkin autoubiquitination assay was performed in the presence of different doses of E1 (i.e. 1.6, 5 or 10 µg/ml), in the presence or absence of E2 (as indicated) and visualized by means of anti-parkin and anti-FK2 immunoblotting.
Figure 2. E2-independent activity is specific to parkin and is dependent on RING2 integrity.
(A) In vitro ubiquitination reaction products generated by MBP-TRAF6, Rma or Momo in the absence of E1, E2 or E3, or in the presence of all three components including their respective cognate E2 (Full) were subjected to immunoblotting with anti-MBP and anti-FK2, as indicated. Unlike parkin, none of these E3 ligase could catalyze E2-independent ubiquitination. (B) Reaction products generated by disease-associated MBP-parkin K211N and G430D mutants in the absence of E1, E2 or E3, or in the presence of all three components (Full) were subjected to immunoblotting with anti-parkin and anti-FK2, as indicated. Reaction products catalyzed by wild type MBP-parkin in the presence or absence of UbcH7 were immunoblotted alongside for comparison.
Figure 3. MS analysis of reaction products catalyzed by MBP-parkin or IBR-R2.
(A) CBB-stained gel showing the reaction products produced by MBP-parkin or IBR-R2 under different conditions, as indicated. (B) Schematic diagrams of b and y ion assignments of K48- and K63-linkage ubiquitin peptides (C-E) MS/MS analysis of K48- or K63-linked ubiquitin chains catalyzed by IBR-R2. Precursor peaks estimated as K48- and K63-linkages were subsequently confirmed by MS/MS analysis.
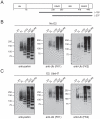
Figure 4. N-terminal region of parkin represses its intrinsic polyubquitination activity.
(A) Schematic depiction of full length parkin protein and the deletion mutants Δ152 and Δ237. (B) In vitro ubiquitination reaction products generated by MBP-parkin, Δ152, Δ237 or IBR-R2 in the absence of E2 were subjected to immunoblotting with anti-parkin, anti-FK1 and anti-FK2, as indicated. (C) As in (B) except that reactions were conducted in the presence of UbcH7.
Figure 5. Parkin S/T-D phospho-mimetic mutants display similar ubiquitination profile as the wild type protein.
(A) Schematic depiction of full length parkin protein with the position of known S/T phosphorylation sites indicated by arrows (B) In vitro ubiquitination reaction products generated by MBP-parkin, MBP-parkin T175D or IBR-R2 in the presence of UbcH7 or Ubc13 were subjected to immunoblotting with anti-parkin, anti-FK1 and anti-FK2, as indicated. (C) In vitro ubiquitination reaction products generated by various parkin phospomimetic mutants in the absence or presence of E2 (UbcH7) were subjected to immunoblotting with anti-parkin and anti-FK1.
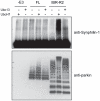
Figure 6. IBR-R2 but not full length parkin promotes synphilin-1ubiquitination.
In vitro ubiquitination reaction was carried out with synphilin-1 as a substrate in the absence of parkin (i.e. –E3) or in the presence of MBP-parkin (FL) or IBR-R2 in combination with either UbcH7, Ubc13 or no E2 (as indicated). Parkin-mediated ubiquitination of synphilin-1 was analyzed by means of anti-synphilin-1 immunoblotting (top panel) whereas parkin-mediated self ubiquitination was analyzed by means of anti-parkin immunoblotting (bottom panel).
Similar articles
- Atypical Ubiquitination and Parkinson's Disease.
Buneeva O, Medvedev A. Buneeva O, et al. Int J Mol Sci. 2022 Mar 28;23(7):3705. doi: 10.3390/ijms23073705. Int J Mol Sci. 2022. PMID: 35409068 Free PMC article. Review. - Parkin mediates the degradation-independent ubiquitination of Hsp70.
Moore DJ, West AB, Dikeman DA, Dawson VL, Dawson TM. Moore DJ, et al. J Neurochem. 2008 Jun;105(5):1806-19. doi: 10.1111/j.1471-4159.2008.05261.x. Epub 2008 Feb 1. J Neurochem. 2008. PMID: 18248624 Free PMC article. - Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation.
Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM. Lim KL, et al. J Neurosci. 2005 Feb 23;25(8):2002-9. doi: 10.1523/JNEUROSCI.4474-04.2005. J Neurosci. 2005. PMID: 15728840 Free PMC article. - Impact of altered phosphorylation on loss of function of juvenile Parkinsonism-associated genetic variants of the E3 ligase parkin.
Aguirre JD, Dunkerley KM, Lam R, Rusal M, Shaw GS. Aguirre JD, et al. J Biol Chem. 2018 Apr 27;293(17):6337-6348. doi: 10.1074/jbc.RA117.000605. Epub 2018 Mar 12. J Biol Chem. 2018. PMID: 29530980 Free PMC article. - Parkin-mediated lysine 63-linked polyubiquitination: a link to protein inclusions formation in Parkinson's and other conformational diseases?
Lim KL, Dawson VL, Dawson TM. Lim KL, et al. Neurobiol Aging. 2006 Apr;27(4):524-9. doi: 10.1016/j.neurobiolaging.2005.07.023. Epub 2005 Oct 6. Neurobiol Aging. 2006. PMID: 16213628 Review.
Cited by
- Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation.
Iguchi M, Kujuro Y, Okatsu K, Koyano F, Kosako H, Kimura M, Suzuki N, Uchiyama S, Tanaka K, Matsuda N. Iguchi M, et al. J Biol Chem. 2013 Jul 26;288(30):22019-32. doi: 10.1074/jbc.M113.467530. Epub 2013 Jun 10. J Biol Chem. 2013. PMID: 23754282 Free PMC article. - The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension.
Smit JJ, Monteferrario D, Noordermeer SM, van Dijk WJ, van der Reijden BA, Sixma TK. Smit JJ, et al. EMBO J. 2012 Oct 3;31(19):3833-44. doi: 10.1038/emboj.2012.217. Epub 2012 Aug 3. EMBO J. 2012. PMID: 22863777 Free PMC article. - Neuronal surface P antigen (NSPA) modulates postsynaptic NMDAR stability through ubiquitination of tyrosine phosphatase PTPMEG.
Espinoza S, Arredondo SB, Barake F, Carvajal F, Guerrero FG, Segovia-Miranda F, Valenzuela DM, Wyneken U, Rojas-Fernández A, Cerpa W, Massardo L, Varela-Nallar L, González A. Espinoza S, et al. BMC Biol. 2020 Nov 6;18(1):164. doi: 10.1186/s12915-020-00877-2. BMC Biol. 2020. PMID: 33158444 Free PMC article. - Proteasome inhibition promotes Parkin-Ubc13 interaction and lysine 63-linked ubiquitination.
Lim GG, Chew KC, Ng XH, Henry-Basil A, Sim RW, Tan JM, Chai C, Lim KL. Lim GG, et al. PLoS One. 2013 Sep 2;8(9):e73235. doi: 10.1371/journal.pone.0073235. eCollection 2013. PLoS One. 2013. PMID: 24023840 Free PMC article. - Parkin Regulation and Neurodegenerative Disorders.
Zhang CW, Hang L, Yao TP, Lim KL. Zhang CW, et al. Front Aging Neurosci. 2016 Jan 12;7:248. doi: 10.3389/fnagi.2015.00248. eCollection 2015. Front Aging Neurosci. 2016. PMID: 26793099 Free PMC article. Review.
References
- Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, et al. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. - PubMed
- Moore DJ. Parkin: a multifaceted ubiquitin ligase. Biochem Soc Trans. 2006;34:749–753. - PubMed
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. - PubMed
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources