Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of insulin receptor substrate (IRS)-1 and IRS-2 - PubMed (original) (raw)
Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of insulin receptor substrate (IRS)-1 and IRS-2
Zhaoyu Sun et al. Endocrinology. 2011 Aug.
Abstract
The IGFs and the IGF type 1 receptor (IGF-1R) are essential mediators of normal mammary gland development in mice. IGF-I and the IGF-1R have demonstrated functions in formation and proliferation of terminal end buds and in ductal outgrowth and branching during puberty. To study the functions of IGF-1R during pregnancy and lactation, we established transgenic mouse lines expressing a human dominant-negative kinase dead IGF-1R (dnhIGF-1R) under the control of the whey acidic protein promoter. We provide evidence that the IGF-1R pathway is necessary for normal epithelial proliferation and alveolar formation during pregnancy. Furthermore, we demonstrate that the whey acidic protein-dnhIGF-1R transgene causes a delay in alveolar differentiation including lipid droplet formation, lumen expansion, and β-casein protein expression. Analysis of IGF-1R signaling pathways showed a decrease in P-IGF-1R and P-Akt resulting from expression of the dnhIGF-1R. We further demonstrate that disruption of the IGF-1R decreases mammary epithelial cell expression of the signaling intermediates insulin receptor substrate (IRS)-1 and IRS-2. No alterations were observed in downstream signaling targets of prolactin and progesterone, suggesting that activation of the IGF-1R may directly regulate expression of IRS-1/2 during alveolar development and differentiation. These data show that IGF-1R signaling is necessary for normal alveolar proliferation and differentiation, in part, through induction of signaling intermediates that mediate alveolar development.
Figures
Fig. 1.
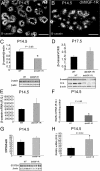
WAP-dnhIGF-1R expression decreases mammary ductal branch outgrowth and alveolar density during pregnancy. A and B, Whole-mount stained glands at P10.5 showing no morphological difference between wild-type (WT) glands and WAP-dnhIGF-1R glands. C and D, Whole-mount stained glands from wild-type and WAP-dnhIGF-1R glands at P14.5. E–H, H&E-stained sections from wild-type and WAP-dnhIGF-1R glands at P14.5 (E and F) and P17.5 (G and H). I, Western blot analysis and quantification of CK8 expression in protein extracted from wild-type or WAP-dnhIGF-1R whole glands at P14.5, P17.5, and L5. Graph shows levels of CK8 adjusted to β-actin; n = 3 animals from each genotype; *, P < 0.03. Scale bars, 100 μm in A–D; 40 μm in E–I.
Fig. 2.
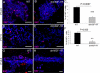
WAP-dnhIGF-1R expression decreases mammary epithelial cell proliferation during pregnancy. A, B, D, and E, Representative photomicrographs used for quantification of Ki67-positive cells in wild-type (WT) glands (A and D) and WAP-dnhIGF-1R glands (B and E) at P14.5 (A and B) and P17.5 (D and E). DAPI was used to detect nuclei (blue). C and F, Quantification of Ki67-positive cells at P14.5 (C) and P17.5 (F) expressed as a percentage of DAPI-positive epithelial cells. G and H, Immunofluorescent images showing reduced Ki67 staining along ducts at sties of alveolar bud outgrowths at P14.5. Scale bars, 50 μm (red autofluorescence of blood cells seen in panel G). **, P < 0.01; ***, P < 0.01.
Fig. 3.
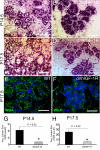
WAP-dnhIGF-1R expression causes deficits in alveolar differentiation during pregnancy. A–D, Representative images of H&E-stained alveoli from wild-type (WT) (A and C) or dnhIGF-1R (B and D) glands at P14.5 (A and B) and at P17.5 (C and D). E and F, Images showing immunofluorescence for WGA (green) and DAPI (blue) in wild-type (E) or dnhIGF-1R (F) glands at P17.5. G and H, Quantification of alveolar lumen size at P14.5 and P17.5. Scale bar, 50 μm. *, P < 0.03.
Fig. 4.
WAP-dnhIGF-1R expression alters myoepithelial cell morphology and CK14 expression but does not effect E-cadherin and ZO-1 expression in alveoli. A and B, Immunofluorescence for CK14 in wild-type (WT) (A) and dnhIGF-1R (B) glands at P17.5. C, Western blot analysis of CK14 expression in isolated MECs from wild-type and dnhIGF-1R glands at P17.5 after adjusting to levels of β-actin. D–G, Immunofluorescence for E-cadherin (E-cad; red, D and E) or ZO-1 (green; F and G) on sections from wild-type (D and F) or dnhIGF-1R (E and G) glands at P17.5. Sections were stained with DAPI to detect nuclei (blue). Scale bars, 50 μm. *, P < 0.05.
Fig. 5.
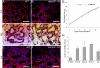
WAP-dnhIGF-1R expression delays alveolar differentiation during pregnancy but does not effect pup weight gain during lactation. A–D, Sections from wild-type (WT) (A and C) and dnhIGF-1R (B and D) glands at L2 were analyzed for lipid droplet formation by ADPH immunofluorescence (red, A and B) or by H&E-staining (C and D). E and F, ADPH immunofluorescence (red) in sections from wild-type (E) and dnhIGF-1R (F) glands at L5. G, Analysis of average pup weight gain from PPD 2–PPD 19 litters from wild-type (n = 5) and dnhIGF-1R (n = 14) dams. H, Relative Q-PCR analysis showing human IGF-1R transgene expression in purified MECs during pregnancy at P10.5, P12.5, P14.5, P17.5, and lactation at L5 after normalization to β-actin. n = 2 for P10.5 and n = 3 for all other time points. Scale bars in A–F, 50 μm. DAPI (blue) was used to detect nuclei in A and B and E and F.
Fig. 6.
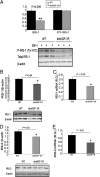
WAP-dnhIGF-1R expression reduces P-IGF-1R and P-Akt in late pregnant MECs. A–D, Immunoprecipitation using anti-P-tyrosine (100) and Western blot analyses to detect IGF-1Rβ (A and B) or IRβ (C and D) after acute (15 min) IGF-I or insulin stimulation of purified MECs from midpregnant (P12.5–P14.5; A and C) and late pregnant (P15.5–P18.5; B and D) glands. Graphs show quantification after normalization to IgG bands. E and F, Western blot analysis and quantification of P-Akt/total Akt expression in MEC samples shown in panels A–D. Control samples were treated with saline in the absence of ligand. n = 3 for each genotype. For midpregnant samples, each lane represents MECs isolated from one to two animals; for late-pregnant samples, each lane represents MECs isolated from individual animals. Panels B and F, *, P < 0.03; panel C, *, P < 0.02. WT, Wild type.
Fig. 7.
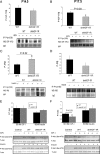
WAP-dnhIGF-1R expression decreases IRS-1 and IRS-2 protein and mRNA expression at P14.5. A, Western blot analysis and quantification of total IRS-1 and P-IRS-1(tyr612)/total IRS-1 in IGF-I stimulated MECs from P12.5–P14.5. B and C, Western blot analysis and quantification of protein levels (B) and Q-PCR analysis of mRNA expression (C) of IRS-1 in isolated MECs at P14.5 after normalization to β-actin. D and E, Western blot analysis and quantification of protein levels (D) and Q-PCR analysis of mRNA expression (E) of IRS-2 in isolated MECs at P14.5 after normalization to β-actin. n = 3 for each genotype; **, P < 0.008 (A); *, P < 0.04 (B); *, P < 0.03 (C); *, P < 0.02 (D); *, P < 0.05 (E). WT, Wild type.
Fig. 8.
WAP-dnhIGF-1R expression decreases β-casein protein expression and STAT5A mRNA expression at P14.5. A and B, Immunofluorescence staining for β-casein on sections from wild-type (WT) (A) and dnhIGF-1R (B) glands at P14.5. C and D, Western blot analysis and quantification of β-casein protein in whole glands at P14.5 after normalization to β-actin expression (C) and at P17.5 after normalization to CK8 to adjust for reduced number of epithelial cells at this time (D). E and F, Q-PCR analysis of β-casein (F) and STAT5A (G) mRNA expression in isolated MECs at P14.5 after normalization to β-actin. G, Western blot analysis and quantification of STAT5A protein in isolated MECs at P14.5 after normalization to β-actin expression. H, Q-PCR analysis of RANKL mRNA expression in isolated MECs at P14.5 after normalization to β-actin. n = 3 for each genotype; *, P < 0.05 (C and F); *, P < 0.02 (H).
Similar articles
- Inhibition of insulin-like growth factor signaling pathways in mammary gland by pure antiestrogen ICI 182,780.
Chan TW, Pollak M, Huynh H. Chan TW, et al. Clin Cancer Res. 2001 Aug;7(8):2545-54. Clin Cancer Res. 2001. PMID: 11489838 - IGF-1R, a target of let-7b, mediates crosstalk between IRS-2/Akt and MAPK pathways to promote proliferation of oral squamous cell carcinoma.
Gao L, Wang X, Wang X, Zhang L, Qiang C, Chang S, Ren W, Li S, Yang Y, Tong D, Chen C, Li Z, Song T, Zhi K, Huang C. Gao L, et al. Oncotarget. 2014 May 15;5(9):2562-74. doi: 10.18632/oncotarget.1812. Oncotarget. 2014. PMID: 24810113 Free PMC article. - Local insulin-like growth factor-II mediates prolactin-induced mammary gland development.
Hovey RC, Harris J, Hadsell DL, Lee AV, Ormandy CJ, Vonderhaar BK. Hovey RC, et al. Mol Endocrinol. 2003 Mar;17(3):460-71. doi: 10.1210/me.2002-0214. Epub 2002 Dec 23. Mol Endocrinol. 2003. PMID: 12554791 - The insulin-like growth factors (IGFs) and IGF binding proteins in postnatal development of murine mammary glands.
Wood TL, Richert MM, Stull MA, Allar MA. Wood TL, et al. J Mammary Gland Biol Neoplasia. 2000 Jan;5(1):31-42. doi: 10.1023/a:1009511131541. J Mammary Gland Biol Neoplasia. 2000. PMID: 10791766 Review. - PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Effects of insulin on mammary gland differentiation during pregnancy and lactation.
Cohick WS. Cohick WS. J Anim Sci. 2016 May;94(5):1812-20. doi: 10.2527/jas.2015-0085. J Anim Sci. 2016. PMID: 27285678 Review.
Cited by
- Breast tumor IGF1R regulates cell adhesion and metastasis: alignment of mouse single cell and human breast cancer transcriptomics.
Obr AE, Bulatowicz JJ, Chang YJ, Ciliento V, Lemenze A, Maingrette K, Shang Q, Gallagher EJ, LeRoith D, Wood TL. Obr AE, et al. Front Oncol. 2022 Dec 7;12:990398. doi: 10.3389/fonc.2022.990398. eCollection 2022. Front Oncol. 2022. PMID: 36568144 Free PMC article. - Mammary alveolar cell as in vitro evaluation system for casein gene expression involved in glucose level.
Heo YT, Ha WT, Lee R, Lee WY, Jeong HY, Hwang KC, Song H. Heo YT, et al. Asian-Australas J Anim Sci. 2017 Jun;30(6):878-885. doi: 10.5713/ajas.16.0515. Epub 2016 Sep 19. Asian-Australas J Anim Sci. 2017. PMID: 27660020 Free PMC article. - Lactational Amenorrhea: Neuroendocrine Pathways Controlling Fertility and Bone Turnover.
Calik-Ksepka A, Stradczuk M, Czarnecka K, Grymowicz M, Smolarczyk R. Calik-Ksepka A, et al. Int J Mol Sci. 2022 Jan 31;23(3):1633. doi: 10.3390/ijms23031633. Int J Mol Sci. 2022. PMID: 35163554 Free PMC article. Review. - Development of endometrial carcinoma in a patient with leprechaunism (donohue syndrome).
Jo W, Sudo S, Nakamura A, Endo D, Konno Y, Ishizu K, Tajima T. Jo W, et al. Clin Pediatr Endocrinol. 2013 Apr;22(2):33-8. doi: 10.1292/cpe.22.33. Epub 2013 Apr 26. Clin Pediatr Endocrinol. 2013. PMID: 23990696 Free PMC article. - Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology.
Lee S, Kelleher SL. Lee S, et al. Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E405-22. doi: 10.1152/ajpendo.00495.2015. Epub 2016 Jun 28. Am J Physiol Endocrinol Metab. 2016. PMID: 27354238 Free PMC article. Review.
References
- Walden PD, Ruan W, Feldman M, Kleinberg DL. 1998. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology 139:659–662 - PubMed
- Ruan W, Kleinberg DL. 1999. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology 140:5075–5081 - PubMed
- Richert MM, Wood TL. 1999. The insulin-like growth factors (IGF) and IGF type I receptor during postnatal growth of the murine mammary gland: sites of messenger ribonucleic acid expression and potential functions. Endocrinology 140:454–461 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous