Mechanisms of lipotoxicity in NAFLD and clinical implications - PubMed (original) (raw)
Review
Mechanisms of lipotoxicity in NAFLD and clinical implications
Samar H Ibrahim et al. J Pediatr Gastroenterol Nutr. 2011 Aug.
Abstract
With the epidemic of childhood obesity, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in pediatrics. NAFLD is strongly associated with insulin resistance and increased level of serum free fatty acids (FFAs). FFAs have direct hepatotoxicity through the induction of an endoplasmic reticulum stress response and subsequently activation of the mitochondrial pathway of cell death. FFAs may also result in lysosomal dysfunction and alter death receptor gene expression. Lipoapoptosis is a key pathogenic process in NAFLD, and correlates with progressive inflammation, and fibrosis. Accumulation of triglyceride in the liver results from uptake and esterification of FFAs by the hepatocyte, and is less likely to be hepatotoxic per se. To date, there are no proven effective therapies that halt NAFLD progression or unfortunately improve prognosis in children. The cellular mechanisms of lipotoxicity are complex but provide potential therapeutic targets for NAFLD. In this review we discuss several potential therapeutic opportunities in detail including inhibition of apoptosis, c-Jun-N-terminal kinase, and endoplasmic reticulum stress pathways.
Figures
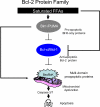
Figure 1. Bcl-2 Protein Family
Saturated FFAs activate the BH3-only proteins (BIM and PUMA), resulting in inactivation of the antiapoptotic Bcl-2 family members (Mcl-1 and Bcl-xL), releasing Bax and Bak from the inhibitory effect of the antiapoptotic Bcl-2 proteins, and causing their activation. Bim and PUMA can also activate Bax and/or Bak directly. Once activated, Bax and Bak promote mitochondrial dysfunction, leading to the activation of the caspase cascade and apoptosis.
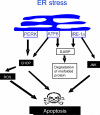
Figure 2. ER stress
In the setting of ER stress the three trans-membrane biosensors PERK, ATF6, and IRE1-a are activated. PERK activation induces the expression of the proapoptotic transcription factor CHOP. CHOP in turn, mediates apoptosis through several pathways including generation of ROS. IRE1-a activates JNK a key player in apoptosis. IRE1-a also generates a spliced form of XBP (s-XBP) that promotes degradation of misfolded proteins. ATF6 contributes in CHOP induction, and heterodimerizes with XBP, enhancing protein degradation.
Figure 3. Integrated model of lipoapotosis by saturated FFAs and potential antiapoptotic agents
Saturated FFAs induce ER stress which in turn activates JNK and CHOP. JNK leads to the upregulation of the pro-apoptotic BH3-only proteins PUMA. CHOP enhances the expression of the proapoptotic BH3-only protein Bim, contributes to PUMA upregulation and mediates the generation of ROS. Bim in cooperation with PUMA induces the activation of the multi-domain executioner proapoptotic protein Bax. Bax activation results in mitochondrial dysfunction, activation of the caspase cascade, and cell death. Saturated FFAs also cause Bax dependent lysosomal permeabilization and release of cathepsin B into the cytosol, cathepsin B mediates down stream mitochondrial permeabilization and apoptosis. Therapeutic strategies to prevent cell death in the setting of saturated FFA-induced apoptosis include as outlined: PUFA, ER Chaperons, GSK3-inhibition, JNK-inhibition, antioxidants, cathepsin B-inhibition, and caspase-inhibition.
Similar articles
- Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease.
Malhi H, Gores GJ. Malhi H, et al. Semin Liver Dis. 2008 Nov;28(4):360-9. doi: 10.1055/s-0028-1091980. Epub 2008 Oct 27. Semin Liver Dis. 2008. PMID: 18956292 Free PMC article. Review. - Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications.
Cusi K. Cusi K. Gastroenterology. 2012 Apr;142(4):711-725.e6. doi: 10.1053/j.gastro.2012.02.003. Epub 2012 Feb 8. Gastroenterology. 2012. PMID: 22326434 Review. - Hepatitis C Virus Infection Increases c-Jun N-Terminal Kinase (JNK) Phosphorylation and Accentuates Hepatocyte Lipoapoptosis.
Takaki H, Akazawa Y, Kido Y, Morishita M, Honda T, Shibata H, Miuma S, Miyaaki H, Taura N, Kondo H, Nakao K. Takaki H, et al. Med Sci Monit. 2017 Sep 21;23:4526-4532. doi: 10.12659/msm.903210. Med Sci Monit. 2017. PMID: 28931802 Free PMC article. - JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease.
Tarantino G, Caputi A. Tarantino G, et al. World J Gastroenterol. 2011 Sep 7;17(33):3785-94. doi: 10.3748/wjg.v17.i33.3785. World J Gastroenterol. 2011. PMID: 21987620 Free PMC article. Review. - Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis.
Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Akazawa Y, et al. J Hepatol. 2010 Apr;52(4):586-93. doi: 10.1016/j.jhep.2010.01.003. Epub 2010 Feb 13. J Hepatol. 2010. PMID: 20206402 Free PMC article.
Cited by
- Lipotoxicity: effects of dietary saturated and transfatty acids.
Estadella D, da Penha Oller do Nascimento CM, Oyama LM, Ribeiro EB, Dâmaso AR, de Piano A. Estadella D, et al. Mediators Inflamm. 2013;2013:137579. doi: 10.1155/2013/137579. Epub 2013 Jan 31. Mediators Inflamm. 2013. PMID: 23509418 Free PMC article. - Emerging role of nanotechnology in treatment of non-alcoholic fatty liver disease (NAFLD).
Moghtadaie A, Mahboobi H, Fatemizadeh S, Kamal MA. Moghtadaie A, et al. EXCLI J. 2023 Sep 4;22:946-974. doi: 10.17179/excli2023-6420. eCollection 2023. EXCLI J. 2023. PMID: 38023570 Free PMC article. Review. - Exploring the genetic basis of fatty liver development in geese.
Yang Y, Wang H, Li G, Liu Y, Wang C, He D. Yang Y, et al. Sci Rep. 2020 Aug 31;10(1):14279. doi: 10.1038/s41598-020-71210-8. Sci Rep. 2020. PMID: 32868783 Free PMC article. - Association between cagA negative Helicobacter pylori status and nonalcoholic fatty liver disease among adults in the United States.
Kang SJ, Kim HJ, Kim D, Ahmed A. Kang SJ, et al. PLoS One. 2018 Aug 15;13(8):e0202325. doi: 10.1371/journal.pone.0202325. eCollection 2018. PLoS One. 2018. PMID: 30110395 Free PMC article. - Hepatocytic Ballooning in Non-alcoholic Steatohepatitis: Bridging the Knowledge Gap and Charting Future Avenues.
Singla T, Muneshwar KN, Pathade AG, Yelne S. Singla T, et al. Cureus. 2023 Sep 25;15(9):e45884. doi: 10.7759/cureus.45884. eCollection 2023 Sep. Cureus. 2023. PMID: 37885505 Free PMC article. Review.
References
- Schwimmer JB, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. - PubMed
- Xanthakos S, et al. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4(2):226–32. - PubMed
- Powell EE, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. - PubMed
- Propst A, et al. Prognosis in nonalcoholic steatohepatitis. Gastroenterology. 1995;108(5):1607. - PubMed
- Adams LA, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK041876/DK/NIDDK NIH HHS/United States
- DK41876/DK/NIDDK NIH HHS/United States
- K08 DK084310-01/DK/NIDDK NIH HHS/United States
- K08 DK084310/DK/NIDDK NIH HHS/United States
- R37 DK041876/DK/NIDDK NIH HHS/United States
- K08 DK084310-02/DK/NIDDK NIH HHS/United States
- R01 DK041876-24/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous