Distance burning: how gut microbes promote extraintestinal cancers - PubMed (original) (raw)
Distance burning: how gut microbes promote extraintestinal cancers
Arlin B Rogers. Gut Microbes. 2011 Jan-Feb.
Abstract
Gut microbes play a major role in carcinogenesis of the gastrointestinal tract. We and others have shown in mouse models that colonic bacteria also influence the development of extraintestinal cancers including hepatocellular and mammary carcinomas. Microbes such as Helicobacter hepaticus invoke a proinflammatory microenvironment in the lower bowel that may extend to distant organs, often in the absence of histologically evident inflammation. Innate immunity plays a crucial role in the promotion of liver cancer and other systemic diseases by gut microbes. Additional mechanisms include type 1 adaptive immunity, altered metabolism, and oxidative stress. Emerging links between host genetics, gut microbes, inflammatory bowel disease and colorectal cancer also may prove useful for the correlation of specific bacterial populations with extraintestinal neoplasms. Interruption of deleterious host-microbe networks through judicious use of antibiotics and targeted molecular therapies may help reduce the incidence of liver, breast, and other human cancers.
© 2011 Landes Bioscience
Figures
Figure 1
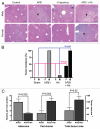
Enterically confined H. hepaticus promoted aflatoxin B1-induced liver cancer in mice in association with NFκB activation. (A) Liver histopathology of male (top row) and female (bottom row) mice in the 4 treatment groups; *adenoma, †HCC. (B) Tumor incidence comparison in male and female mice from each treatment group. (C) Adenoma versus carcinoma multiplicity and total tumor surface area comparison in AFB1-exposed males with and without H. hepaticus. (D) Pathways analysis of NFκB-anchored molecular networks altered by H. hepaticus in both the lower bowel and liver. Images reproduced from Fox et al. Gut 2010. {Fox 2010 #4672}.
Figure 1
Enterically confined H. hepaticus promoted aflatoxin B1-induced liver cancer in mice in association with NFκB activation. (A) Liver histopathology of male (top row) and female (bottom row) mice in the 4 treatment groups; *adenoma, †HCC. (B) Tumor incidence comparison in male and female mice from each treatment group. (C) Adenoma versus carcinoma multiplicity and total tumor surface area comparison in AFB1-exposed males with and without H. hepaticus. (D) Pathways analysis of NFκB-anchored molecular networks altered by H. hepaticus in both the lower bowel and liver. Images reproduced from Fox et al. Gut 2010. {Fox 2010 #4672}.
Figure 2
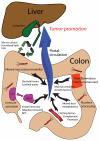
Potential mechanisms of liver tumor promotion by gut microbes. Intestinal bacteria may promote hepatic (and other) distant neoplasms through at least six nonexclusive pathways: (1) altered mucosal permeability with toxin absorption; (2) induction of proinflammatory mucosal/epithelial microenvironments; (3) generalized immune activation; (4) activation of circulating proteases and other serum factors; (5) bacterial nutrient processing and host metabolomics; and (6) disrupted bile and cholesterol/fatty acid homeostasis. APC, antigen-presenting cells; FGFs, fibroblast growth factors; GALT, gut-associated lymphoid tissue; MLN, mesenteric lymph node.
Comment on
- Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens.
Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, Williams M, McFaline JL, Essigmann JM, Schauer DB, Tannenbaum SR, Dedon PC, Weinman SA, Lemon SM, Fry RC, Rogers AB. Fox JG, et al. Gut. 2010 Jan;59(1):88-97. doi: 10.1136/gut.2009.183749. Gut. 2010. PMID: 19850960 Free PMC article.
Similar articles
- Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens.
Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, Williams M, McFaline JL, Essigmann JM, Schauer DB, Tannenbaum SR, Dedon PC, Weinman SA, Lemon SM, Fry RC, Rogers AB. Fox JG, et al. Gut. 2010 Jan;59(1):88-97. doi: 10.1136/gut.2009.183749. Gut. 2010. PMID: 19850960 Free PMC article. - Mining the Human Gut Microbiota for Immunomodulatory Organisms.
Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, Benoist C, Kasper DL. Geva-Zatorsky N, et al. Cell. 2017 Feb 23;168(5):928-943.e11. doi: 10.1016/j.cell.2017.01.022. Epub 2017 Feb 16. Cell. 2017. PMID: 28215708 Free PMC article. - Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice.
Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Rao VP, et al. Cancer Res. 2006 Aug 1;66(15):7395-400. doi: 10.1158/0008-5472.CAN-06-0558. Cancer Res. 2006. PMID: 16885333 - The microbiome and hepatobiliary-pancreatic cancers.
Mima K, Nakagawa S, Sawayama H, Ishimoto T, Imai K, Iwatsuki M, Hashimoto D, Baba Y, Yamashita YI, Yoshida N, Chikamoto A, Baba H. Mima K, et al. Cancer Lett. 2017 Aug 28;402:9-15. doi: 10.1016/j.canlet.2017.05.001. Epub 2017 May 17. Cancer Lett. 2017. PMID: 28527946 Review. - Breast cancer: should gastrointestinal bacteria be on our radar screen?
Rao VP, Poutahidis T, Fox JG, Erdman SE. Rao VP, et al. Cancer Res. 2007 Feb 1;67(3):847-50. doi: 10.1158/0008-5472.CAN-06-3468. Cancer Res. 2007. PMID: 17283110 Review.
Cited by
- The gut-liver axis and the intersection with the microbiome.
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. Tripathi A, et al. Nat Rev Gastroenterol Hepatol. 2018 Jul;15(7):397-411. doi: 10.1038/s41575-018-0011-z. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29748586 Free PMC article. Review. - Food System Transformation and Gut Microbiota Transition: Evidence on Advancing Obesity, Cardiovascular Diseases, and Cancers-A Narrative Review.
Elechi JOG, Sirianni R, Conforti FL, Cione E, Pellegrino M. Elechi JOG, et al. Foods. 2023 Jun 6;12(12):2286. doi: 10.3390/foods12122286. Foods. 2023. PMID: 37372497 Free PMC article. Review. - Evolutionary foundations for cancer biology.
Aktipis CA, Nesse RM. Aktipis CA, et al. Evol Appl. 2013 Jan;6(1):144-59. doi: 10.1111/eva.12034. Epub 2013 Jan 21. Evol Appl. 2013. PMID: 23396885 Free PMC article. - Publisher Correction: The gut-liver axis and the intersection with the microbiome.
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. Tripathi A, et al. Nat Rev Gastroenterol Hepatol. 2018 Dec;15(12):785. doi: 10.1038/s41575-018-0031-8. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29785003 Free PMC article. - TLR4 Deficiency Protects against Hepatic Fibrosis and Diethylnitrosamine-Induced Pre-Carcinogenic Liver Injury in Fibrotic Liver.
Weber SN, Bohner A, Dapito DH, Schwabe RF, Lammert F. Weber SN, et al. PLoS One. 2016 Jul 8;11(7):e0158819. doi: 10.1371/journal.pone.0158819. eCollection 2016. PLoS One. 2016. PMID: 27391331 Free PMC article.
References
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. - PubMed
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. - PubMed
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical