Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier - PubMed (original) (raw)
Review
Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier
Marianne Strazza et al. Brain Res. 2011.
Abstract
Human immunodeficiency virus type 1 (HIV-1) primarily infects CD4(+) T cells and cells of the monocyte-macrophage lineage, resulting in immunodeficiency in an infected patient. Along with this immune deficiency, HIV-1 has been linked to a number of neurological symptoms in the absence of opportunistic infections or other co-morbidities, suggesting that HIV-1 is able to cross the blood-brain barrier (BBB), enter the central nervous system (CNS), and cause neurocognitive impairment. HIV-1-infected monocyte-macrophages traverse the BBB and enter the CNS throughout the course of HIV-1 disease. Once in the brain, both free virus and virus-infected cells are able to infect neighboring resident microglia and astrocytes and possibly other cell types. HIV-1-infected cells in both the periphery and the CNS give rise to elevated levels of viral proteins, including gp120, Tat, and Nef, and of host inflammatory mediators such as cytokines and chemokines. It has been shown that the viral proteins may act alone or in concert with host cytokines and chemokines, affecting the integrity of the BBB. The pathological end point of these interactions may facilitate a positive feedback loop resulting in increased penetration of HIV into the CNS. It is proposed in this review that the dysregulation of the BBB during and after neuroinvasion is a critical component of the neuropathogenic process and that dysregulation of this protective barrier is caused by a combination of viral and host factors including secreted viral proteins, components of the inflammatory process, the aging process, therapeutics, and drug or alcohol abuse.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
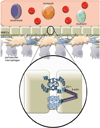
Fig. 1. The physiologically normal blood-brain barrier (BBB)
The BBB is made up of specialized endothelial cells, brain microvascular endothelial cells (BMECs), surrounded by astrocytes and pericytes, which are involved in the creation of the biochemical environment. The BBB functions to separate peripheral circulation from the central nervous system (CNS). The limited permeability of the barrier allows highly regulated transport of cells and other substances into the CNS. It also separates the CNS from peripheral immune surveillance. Circulating monocytes that gain entry into the CNS develop into resident macrophages. The function of the BBB is established by the tight junctions (TJs) between BMECs. TJ proteins include the transmembrane proteins claudin and occludin along with zonula occludens 1 (ZO-1) which connect the transmembrane proteins to the cytoskeleton.
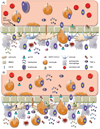
Fig. 2. The effects of HIV proteins on BMECs
(A) Cellular responses to Tat resulting in increased blood-brain barrier (BBB) permeability. Tat activates NF-kappaB and mitogen-activated protein kinase through an unknown mechanism, thereby causing a decrease in TNF-alpha neutralization. The resulting accumulation of TNF-alpha causes an increase in MMP-9, which can function locally or may be secreted into the circulation. It is also possible that NF-kappaB and mitogen-activated protein kinase activity directly causes the increase in MMP-9 levels, but the mechanism is unknown. Active MMP-9 degrades laminin and type IV collagen, essential components of the BBB. Tat has been observed to increase ROS within the cell, an effect amplified in the presence of TNF-alpha and alcohol metabolism. ROS causes increased phosphorylation of occludin, claudin-5, and ZO-1 and causes a general decrease in tight junction (TJ) mRNA and protein levels. Tat and TNF-alpha lead to the activation of COX-2 at caveolae in the cell membrane, leading to a decrease in protein levels of occludin and ZO-1. Tat also activates Ras through an unknown mechanism requiring CAV-1, leading to a decrease in protein levels of occludin, ZO-1, and ZO-2. Overall, occludin, claudin-5, ZO-1, and ZO-2 are mislocalized in the cytoplasm, where they are likely degraded by the proteasome. (B) As gp120 binds to either CXCR4 or CCR5, changes within the cell lead to TJ dysfunction, a decrease in TEER, and an increase in BBB permeability. A resulting increase in intracellular concentrations of Ca2+ and activation of protein kinase C and myosin light chain kinase have been observed following gp120 binding. Surface levels of ICAM-1 and VCAM-1 increase significantly, leading to an increase in recruitment and transendothelial migration of lymphocytes and monocytes from the peripheral circulation. Although protein levels of claudin-1 and -5 are unaffected by gp120, the proteins ZO-1, ZO-2, and occludin are targeted to the proteasome for degradation. With loss of connection to the cytoskeleton, claudin-1 and -5 are unable to maintain TJ function. An increase in intracellular ROS is also observed as a result of decreased levels of glutathione peroxidase and glutathione reductase following exposure to gp120. (C) HIV-1 infection of astrocytes is characterized by expression of Tat, high levels of Nef, and increased secretion of proinflammatory cytokines TNF-alpha and IL-6. Nef expression is associated with astrocyte activation, as measured by increased CD88 surface expression and elevated glial fibrillary acidic protein. Myristoylated Nef anchored to the cell membrane can interact with calmodulin and has been associated with increased secretion of MCP-1/CCL2, a potent monocyte chemoattractant. MCP-1/CCL2 secretion decreases protein levels of occludin, claudin-5, ZO-1, and ZO-2 at the TJs. An increase in secretion of IL-2 and IL-8 has also been observed, along with a decrease in secretion of stromal cell-derived factor-1 and IL-10.
Fig. 3. Tipping point: A model of BBB dysregulation and loss of homeostasis
A) During acute infection, an initial viral entry event into the CNS occurs. This is believed to be through entry of an infected cell of the monocyte-macrophage lineage. Throughout chronic infection, increased generation of or differentiation to a specific monocyte subset, CD14low/CD16+ that traffics preferentially to the BBB and constitutively secretes IL-1 and TNF-alpha is observed in patients with HIV. Once at the BBB, infected cells secrete IL-1, TNF-alpha, Tat, gp120 and other viral proteins causing altered TJ protein expression and localization and BMEC activation leading to increased passage across the BBB. Crosstalk between monocytes and BMECs in this inflammatory state leads to reciprocal activation of the two cell types, allowing for diapedesis of, likely infected, monocytes. Tat and gp120 are able to enter BMECs through adsorptive endocytosis, altering TJ regulation and delaying TJ closure, creating an opportunity for free virus and viral proteins to enter the CNS through non-specific passage mechanisms. In addition, gap formation has been observed under these conditions, however, the endothelium is able to repair. In the CNS, other cells including astrocytes, pericytes, and perivascular macrophages can be infected and will further secrete Tat, gp120, and proinflammatory cytokines leading to further BMEC activation. Additionally, astrocytes will express high levels of Nef, and secrete MCP-1/CCL2, IL-2, 6, and 8. This process greatly facilitates viral entry into the CNS. Additionally, Tat has been shown to be chemotactic for monocytes, bringing more monocytes to the site of infection. While homeostasis is strained, as long as the barrier is able to recover and repair balance can be restored. This state of enhanced passage may correlate with early HAND development. B) After prolonged exposure to viral proteins, proinflammatory cytokines, and infectious virus, BBB dysregulation exceeds the threshold of what can be restored. Constant BMEC activation leads to greatly increased monocyte firm adhesion and diapedesis, further spreading infection throughout the CNS. High levels of Tat and gp120 entering BMECs altering TJ protein expression and stability cause further delays in TJ closing, and there is more leakiness in passage. Gap formation is also enhanced, pushing repair mechanisms to a limit. Astrocyte death is also observed, often in cells neighboring those that are infected. Disturbance of the homeostasis between the periphery and the CNS, along with peaks in inflammatory cytokines, neurotoxic viral proteins, and infectious virions suggest a correlation with the onset of more severe neurocognitive symptoms.
Similar articles
- Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications.
Hong S, Banks WA. Hong S, et al. Brain Behav Immun. 2015 Mar;45:1-12. doi: 10.1016/j.bbi.2014.10.008. Epub 2014 Oct 22. Brain Behav Immun. 2015. PMID: 25449672 Free PMC article. Review. - Mechanisms of CNS Viral Seeding by HIV+ CD14+ CD16+ Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders.
Veenstra M, León-Rivera R, Li M, Gama L, Clements JE, Berman JW. Veenstra M, et al. mBio. 2017 Oct 24;8(5):e01280-17. doi: 10.1128/mBio.01280-17. mBio. 2017. PMID: 29066542 Free PMC article. - HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis.
Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. Kanmogne GD, et al. J Cereb Blood Flow Metab. 2007 Jan;27(1):123-34. doi: 10.1038/sj.jcbfm.9600330. Epub 2006 May 10. J Cereb Blood Flow Metab. 2007. PMID: 16685256 Free PMC article. - An Elvitegravir Nanoformulation Crosses the Blood-Brain Barrier and Suppresses HIV-1 Replication in Microglia.
Gong Y, Zhi K, Nagesh PKB, Sinha N, Chowdhury P, Chen H, Gorantla S, Yallapu MM, Kumar S. Gong Y, et al. Viruses. 2020 May 20;12(5):564. doi: 10.3390/v12050564. Viruses. 2020. PMID: 32443728 Free PMC article. - CNS inflammation and macrophage/microglial biology associated with HIV-1 infection.
Yadav A, Collman RG. Yadav A, et al. J Neuroimmune Pharmacol. 2009 Dec;4(4):430-47. doi: 10.1007/s11481-009-9174-2. Epub 2009 Sep 19. J Neuroimmune Pharmacol. 2009. PMID: 19768553 Free PMC article. Review.
Cited by
- Neuronal toxicity in HIV CNS disease.
Kovalevich J, Langford D. Kovalevich J, et al. Future Virol. 2012 Jul 1;7(7):687-698. doi: 10.2217/fvl.12.57. Future Virol. 2012. PMID: 23616788 Free PMC article. - Brain microbial populations in HIV/AIDS: α-proteobacteria predominate independent of host immune status.
Branton WG, Ellestad KK, Maingat F, Wheatley BM, Rud E, Warren RL, Holt RA, Surette MG, Power C. Branton WG, et al. PLoS One. 2013;8(1):e54673. doi: 10.1371/journal.pone.0054673. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23355888 Free PMC article. Clinical Trial. - Pericyte infection by HIV-1: a fatal attraction.
Naranjo O, Torices S, Clifford PR, Daftari MT, Osborne OM, Fattakhov N, Toborek M. Naranjo O, et al. Retrovirology. 2022 Dec 7;19(1):27. doi: 10.1186/s12977-022-00614-3. Retrovirology. 2022. PMID: 36476484 Free PMC article. Review. - Neuroimmunometabolic alterations and severity of depressive symptoms in people with HIV: An exploratory diffusion-weighted MRS study.
Mudra Rakshasa-Loots A, Diteko G, Dowell NG, Ronen I, Vera JH. Mudra Rakshasa-Loots A, et al. Brain Neurosci Adv. 2025 Apr 29;9:23982128251335792. doi: 10.1177/23982128251335792. eCollection 2025 Jan-Dec. Brain Neurosci Adv. 2025. PMID: 40308263 Free PMC article. - Nipah Virus Infection of Immature Dendritic Cells Increases Its Transendothelial Migration Across Human Brain Microvascular Endothelial Cells.
Tiong V, Shu MH, Wong WF, AbuBakar S, Chang LY. Tiong V, et al. Front Microbiol. 2018 Nov 13;9:2747. doi: 10.3389/fmicb.2018.02747. eCollection 2018. Front Microbiol. 2018. PMID: 30483242 Free PMC article.
References
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. - PubMed
- Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. - PubMed
- Banks WA, Kastin AJ, Akerstrom V. HIV-1 protein gp120 crosses the blood-brain barrier: role of adsorptive endocytosis. Life Sci. 1997;61:PL119–PL125. - PubMed
- Banks WA, Akerstrom V, Kastin AJ. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. J Cell Sci. 1998;111(Pt 4):533–540. - PubMed
Publication types
MeSH terms
Grants and funding
- DA19807/DA/NIDA NIH HHS/United States
- R01 NS032092-21/NS/NINDS NIH HHS/United States
- NS46263/NS/NINDS NIH HHS/United States
- R01 DA019807-07/DA/NIDA NIH HHS/United States
- NS32092/NS/NINDS NIH HHS/United States
- T32 MH079785-03/MH/NIMH NIH HHS/United States
- T32 MH079785/MH/NIMH NIH HHS/United States
- R01 NS032092/NS/NINDS NIH HHS/United States
- 5T32MH079785/MH/NIMH NIH HHS/United States
- R01 NS046263/NS/NINDS NIH HHS/United States
- R01 DA019807/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials