Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months - PubMed (original) (raw)
Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months
Shawn J Kohler et al. Proc Natl Acad Sci U S A. 2011.
Abstract
We studied two groups of adult macaque monkeys to determine the time course of adult neurogenesis in the dentate gyrus of the hippocampus. In the first group, six adult monkeys (Macaca mulatta) received a single injection of the thymidine analog BrdU (75 mg/kg), which is incorporated into replicating DNA and serves as a marker for new cell birth. Brain tissue was collected 48 h, 2 wk, and 6 wk after BrdU injection to examine the initial stages of neurogenesis. Because mature neurons were not evident at 6 wk, we examined tissue collected over a longer time course in a second study. In this study, eight monkeys (Macaca fascicularis) who were subjects in a separate exercise study received 10 weekly injections of BrdU (75 mg/kg), and brain tissue was collected at 16 and 28 wk from the first injection. Based on the timing of expression of neuronal cell markers (βIII-tubulin, doublecortin, NeuN), the extent of dendritic arborization, and acquisition of mature cell body morphology, we show that granule cell maturation in the dentate gyrus of a nonhuman primate is protracted over a minimum of a 6-mo time period, more than 6 times longer than in rodents. The lengthened time course for new cell maturation in nonhuman primates may be appropriate for preservation of neural plasticity over their longer life span and is relevant to our understanding of antidepressant and other therapies that have been linked to neurogenesis in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
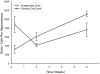
Study 1: Total number of BrdU+ cells born after a single BrdU injection (mean ± SEM). There was an inverse relationship between the numbers of new cells in SGZ (○) and GCL (▽) consistent with new cell migration from the SGZ to GCl during that time period (ANOVA, effect of time, P = 0.020; time vs. layer interaction, P = 0.014). There was also an increase in the number of BrdU+ cells over the 6 wk in study 1 (_r_2 = 0.940, P = 0.001).
Fig. 2.
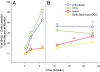
New neurons in the GCL showed a partial transition from immature to mature neuronal markers throughout the time points of study 1 and 2 (mean ± SEM). (A) In study 1, 84% of BrdU+ cells were labeled with the immature marker DCX at 6 wk, whereas only 10% were labeled with the mature marker NeuN. (B) In study 2, there was a significant increase in the number of NeuN+ cells from 11 to 23 wk (*P = 0.048) and at the 23-wk time point, 54% of BrdU+ neurons still maintained expression of the immature marker βIII-tubulin, whereas only 34% were NeuN+ (βIII-tubulin, ○; DCX, ▽; NeuN, □; both NeuN and DCX, ◇).
Fig. 3.
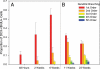
New DCX+ neurons showed a pattern of consistent increase in the number of apical dendritic branches over the length of the study (mean ± SEM). (A) In study 1, at 48 h, only 10% of DCX+ cells had a dendritic process and at 6 wk, 55% of cells still had a single unbranched dendrite. (B) In study 2, at the 11- and 23-wk time points, there were increasing numbers of dendrites with 4–5 branches (first order, red; second order, orange; third order, yellow; fourth order, green; fifth order, blue).
Fig. 4.
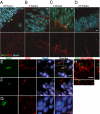
Confocal micrographs demonstrating the sequence of neuron maturation. (A_–_D) Micrographs represent the most mature cells observed at each time point (Upper, an overlay of BrdU and DCX; Lower, the same image showing DCX only). Study 1 (A_–_C). (A) At 48 h, the most mature neurons were weakly DCX positive with a single apical dendrite (scale bar: 10 μm). (B) At 2 wk, the most mature cells had fusiform nuclei and dendrites reaching the edge of the GCL (scale bar: 10 μm). (C) At 6 wk, the most mature neurons had dendrites with 2 or 3 branches (scale bar: 10 μm). (D) In Study 2 at 23 wk, the most mature DCX+ neurons still had fusiform shapes with basilar dendrites (scale bar: 20 μm; BrdU, green; DCX, red; NeuN, blue). (E_–_G) NeuN+/BrdU+ cells showed immature morphology until the 23-wk time point. (E) In study 1 at 6 wk, nuclei and cell bodies of NeuN+ cells were fusiform in shape. However, in study 2 at the 23-wk time point, some NeuN+ cells had round nuclei comparable to mature granule cells and were either weakly labeled (F) or not labeled with DCX (G; scale bar: 10 μm; BrdU, green; DCX, red; NeuN, blue). (H) Confocal micrograph of βIII-tubulin+/BrdU+ cell with oblique views (scale bar: 10 μm).
Similar articles
- Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus.
Jabès A, Lavenex PB, Amaral DG, Lavenex P. Jabès A, et al. Eur J Neurosci. 2010 Jan;31(2):273-85. doi: 10.1111/j.1460-9568.2009.07061.x. Epub 2010 Jan 13. Eur J Neurosci. 2010. PMID: 20074220 Free PMC article. - Adult-generated hippocampal and neocortical neurons in macaques have a transient existence.
Gould E, Vail N, Wagers M, Gross CG. Gould E, et al. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10910-7. doi: 10.1073/pnas.181354698. Epub 2001 Aug 28. Proc Natl Acad Sci U S A. 2001. PMID: 11526209 Free PMC article. - Neurogenesis in the septal and temporal part of the adult rat dentate gyrus.
Bekiari C, Giannakopoulou A, Siskos N, Grivas I, Tsingotjidou A, Michaloudi H, Papadopoulos GC. Bekiari C, et al. Hippocampus. 2015 Apr;25(4):511-23. doi: 10.1002/hipo.22388. Epub 2014 Nov 29. Hippocampus. 2015. PMID: 25394554 - Adult neurogenesis in the mammalian dentate gyrus.
Abbott LC, Nigussie F. Abbott LC, et al. Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review. - Clinical neuropathology practice guide 5-2013: markers of neuronal maturation.
Sarnat HB. Sarnat HB. Clin Neuropathol. 2013 Sep-Oct;32(5):340-69. doi: 10.5414/NP300638. Clin Neuropathol. 2013. PMID: 23883617 Free PMC article. Review.
Cited by
- Hippocampal neurogenesis in adult primates: a systematic review.
Elliott T, Liu KY, Hazan J, Wilson J, Vallipuram H, Jones K, Mahmood J, Gitlin-Leigh G, Howard R. Elliott T, et al. Mol Psychiatry. 2024 Nov 18. doi: 10.1038/s41380-024-02815-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39558003 - Spatial transcriptomic analysis of adult hippocampal neurogenesis in the human brain.
Simard S, Rahimian R, Davoli MA, Théberge S, Matosin N, Turecki G, Nagy C, Mechawar N. Simard S, et al. J Psychiatry Neurosci. 2024 Oct 16;49(5):E319-E333. doi: 10.1503/jpn.240026. Print 2024 Sep-Oct. J Psychiatry Neurosci. 2024. PMID: 39414359 Free PMC article. - Which neurodevelopmental processes continue in humans after birth?
Sorrells SF. Sorrells SF. Front Neurosci. 2024 Sep 6;18:1434508. doi: 10.3389/fnins.2024.1434508. eCollection 2024. Front Neurosci. 2024. PMID: 39308952 Free PMC article. - Transcriptional dynamics orchestrating the development and integration of neurons born in the adult hippocampus.
Rasetto NB, Giacomini D, Berardino AA, Waichman TV, Beckel MS, Di Bella DJ, Brown J, Davies-Sala MG, Gerhardinger C, Lie DC, Arlotta P, Chernomoretz A, Schinder AF. Rasetto NB, et al. Sci Adv. 2024 Jul 19;10(29):eadp6039. doi: 10.1126/sciadv.adp6039. Epub 2024 Jul 19. Sci Adv. 2024. PMID: 39028813 Free PMC article. - Molecular cascade reveals sequential milestones underlying hippocampal neural stem cell development into an adult state.
Jimenez-Cyrus D, Adusumilli VS, Stempel MH, Maday S, Ming GL, Song H, Bond AM. Jimenez-Cyrus D, et al. Cell Rep. 2024 Jun 25;43(6):114339. doi: 10.1016/j.celrep.2024.114339. Epub 2024 Jun 8. Cell Rep. 2024. PMID: 38852158 Free PMC article.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources