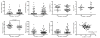Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection - PubMed (original) (raw)
Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection
Jennifer L Lyons et al. J Acquir Immune Defic Syndr. 2011.
Abstract
Objective: Mild forms of HIV-associated neurocognitive disorders (HAND) remain prevalent in the era of combination antiretroviral therapy (cART). Although elevated lipopolysaccharide (LPS) and immune activation are implicated in HAND pathogenesis, relationships of LPS and inflammatory markers to mild forms of HAND or impairment in specific cognitive domains are unknown. To examine these relationships, we compared plasma soluble CD14 (sCD14), CCL2, and LPS levels with neurocognitive test scores in a cART era cohort.
Methods: We analyzed plasma from HIV+ subjects (n = 97) with nadir CD4 counts <300 and high frequency of hepatitis C virus coinfection and illicit drug use for relationships between sCD14, CCL2, and LPS levels and neurocognitive test scores.
Results: Plasma sCD14 levels were higher in subjects with test scores indicating global impairment (P = 0.007), particularly in attention and learning domains (P = 0.015 and P = 0.03, respectively), regardless of HAND diagnosis. Plasma sCD14 levels correlated inversely with global, attention, and learning T scores (P = 0.036, 0.047, and 0.007, respectively) and yielded higher area under receiver operating characteristic values for predicting impaired scores than single-marker models based on plasma or cerebrospinal fluid viral load or CD4 count (area under receiver operating characteristic values = 0.71, 0.81, and 0.71, respectively) and in 4-marker models based on plasma sCD14 and 3 conventional markers compared with the 3-marker models.
Conclusions: Plasma sCD14 is a biomarker associated with impaired neurocognitive testing in attention and learning domains in HIV-infected individuals with advanced disease, suggesting involvement of cortical and limbic pathways by inflammatory processes in the cART era. Plasma sCD14 is a potential biomarker to monitor HAND progression and therapeutic responses.
Conflict of interest statement
The study authors report no disclosures or competing interests.
Figures
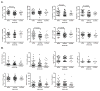
Figure 1. Subjects with more severe HAND diagnoses have lower global T scores
Neurocognitive test scores were compared to each HAND diagnosis in HIV+ subjects. No NCI is followed in severity by ANI, in which subjects demonstrate neurocognitive impairment but have no daily functioning deficits, which in turn is followed in severity by MCMD, where mild neurocognitive impairment is combined with daily functioning deficits, and then HAD, in which patients evidence moderate to severe neurocognitive impairment and daily functioning impairments. Global T scores were lower with diagnoses of increased severity of neurocognitive impairment as compared to no NCI (top left panel). Domain T scores for attention, learning, and memory were significantly lower for all diagnoses of impairment, whereas T scores for fluency, SIP, executive function, and motor were generally associated only with more severe forms of neurocognitive impairment (i.e., HAD and MCMD) and NPI-O. (SIP, speed of information processing; NCI, neurocognitive impairment; ANI, asymptomatic neurocognitive impairment; MCMD, minor cognitive-motor disorder; HAD, HIV-associated dementia; NPI-O, other neuropsychiatric impairment). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure 2. Higher plasma sCD14 levels are associated with global T scores indicating neurocognitive impairment
Subjects were grouped by global T <40 or ≥40 to compare biomarker levels. Only plasma sCD14 levels were significantly different between subjects with versus without impaired global T scores (second row, first panel), and correlated negatively with global T scores (second row, last panel). (VL, viral load). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test, and significance among continuous variables was calculated using Spearman rho correlation; significant differences (p<0.05) are indicated.
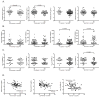
Figure 3. Higher plasma sCD14 levels are associated with impaired test performance in attention and learning domains
Plasma levels of sCD14, CD4 count, and plasma VL were compared to domain T scores in subjects grouped by T scores <40 or ≥40. A. Plasma sCD14 levels were elevated in subjects with attention and learning T scores <40, B. Lower CD4 counts in subjects with fluency and motor T scores <40. C. Higher plasma VL in subjects with motor T scores <40. D. Plasma sCD14 correlated negatively with attention and learning T scores, and plasma VL correlated negatively with motor T scores. (VL, viral load). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test, and correlations between continuous variables were analyzed using Spearman rho correlation; significant differences (p<0.05) are indicated.
Figure 4. HIV+ opiate users demonstrate impairment in global T scores and learning, memory, motor, and SIP domain T scores but no difference in plasma sCD14 levels compared to non-users
Global and domain T scores, as well as plasma biomarker levels and HIV disease markers, were compared between subjects with no current drug use, current opiate use (including 18 subjects using both opiates and cocaine), and current cocaine use. Subjects were categorized as opiate or cocaine users if they endorsed syndromic abuse and/or urine toxicology was positive for opiates (including methadone) or cocaine (n=35 and 22, respectively). Subjects with negative toxicology screening for opiates or cocaine and without PRISM or CIDI diagnosis of substance abuse were categorized as non-users (“None”, n=38). A. Opiate users had lower global T scores than cocaine users or non-users, and lower learning, memory, motor and SIP T scores than non-users. B. Current and nadir CD4 count, plasma VL, CSF VL, and plasma sCD14, CCL2, and LPS levels did not differ between groups. (SIP, speed of information processing; VL, viral load). Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the two-tailed Mann-Whitney test; significant differences (p<0.05) are indicated.
Similar articles
- Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection.
Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Kamat A, et al. J Acquir Immune Defic Syndr. 2012 Jul 1;60(3):234-43. doi: 10.1097/QAI.0b013e318256f3bc. J Acquir Immune Defic Syndr. 2012. PMID: 22569268 Free PMC article. - Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: the Neuradapt study.
Vassallo M, Dunais B, Durant J, Carsenti-Dellamonica H, Harvey-Langton A, Cottalorda J, Ticchioni M, Laffon M, Lebrun-Frenay C, Dellamonica P, Pradier C. Vassallo M, et al. J Neurovirol. 2013 Aug;19(4):376-82. doi: 10.1007/s13365-013-0181-y. Epub 2013 Jul 12. J Neurovirol. 2013. PMID: 23846287 - A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy.
Kamat A, Misra V, Cassol E, Ancuta P, Yan Z, Li C, Morgello S, Gabuzda D. Kamat A, et al. PLoS One. 2012;7(2):e30881. doi: 10.1371/journal.pone.0030881. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363505 Free PMC article. - Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection.
Cassol E, Misra V, Morgello S, Gabuzda D. Cassol E, et al. J Neuroimmune Pharmacol. 2013 Dec;8(5):1087-97. doi: 10.1007/s11481-013-9512-2. Epub 2013 Nov 21. J Neuroimmune Pharmacol. 2013. PMID: 24259252 Free PMC article. Review. - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
- Simian immunodeficiency virus-infected rhesus macaques with AIDS co-develop cardiovascular pathology and encephalitis.
White KS, Walker JA, Wang J, Autissier P, Miller AD, Abuelezan NN, Burrack R, Li Q, Kim WK, Williams KC. White KS, et al. Front Immunol. 2023 Oct 27;14:1240946. doi: 10.3389/fimmu.2023.1240946. eCollection 2023. Front Immunol. 2023. PMID: 37965349 Free PMC article. - Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism.
DʼAntoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, Seyedkazemi S, Nakamoto BK, Walker M, Kallianpur KJ, Ogata-Arakaki D, Ndhlovu LC, Shikuma C. DʼAntoni ML, et al. J Acquir Immune Defic Syndr. 2018 Sep 1;79(1):108-116. doi: 10.1097/QAI.0000000000001752. J Acquir Immune Defic Syndr. 2018. PMID: 29781885 Free PMC article. Clinical Trial. - Heme oxygenase-1 dysregulation in the brain: implications for HIV-associated neurocognitive disorders.
Ambegaokar SS, Kolson DL. Ambegaokar SS, et al. Curr HIV Res. 2014;12(3):174-88. doi: 10.2174/1570162x12666140526122709. Curr HIV Res. 2014. PMID: 24862327 Free PMC article. Review. - Co-receptor signaling in the pathogenesis of neuroHIV.
Nickoloff-Bybel EA, Festa L, Meucci O, Gaskill PJ. Nickoloff-Bybel EA, et al. Retrovirology. 2021 Aug 24;18(1):24. doi: 10.1186/s12977-021-00569-x. Retrovirology. 2021. PMID: 34429135 Free PMC article. Review. - Modifications in acute phase and complement systems predict shifts in cognitive status of HIV-infected patients.
Ubaida-Mohien C, Lamberty B, Dickens AM, Mielke MM, Marcotte T, Sacktor N, Grant I, Letendre S, Franklin D, Cibrowski P, Tharakan R, McArthur JC, Fox H, Haughey NJ. Ubaida-Mohien C, et al. AIDS. 2017 Jun 19;31(10):1365-1378. doi: 10.1097/QAD.0000000000001503. AIDS. 2017. PMID: 28574961 Free PMC article.
References
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010 Jun;67(6):699–714. - PubMed
- Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008 Jun-Jul;16(2):94–98. - PubMed
- Bhaskaran K, Mussini C, Antinori A, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008 Feb;63(2):213–221. - PubMed
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002 Dec;8( Suppl 2):115–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R24 MH059724/MH/NIMH NIH HHS/United States
- U01 MH083500/MH/NIMH NIH HHS/United States
- R24 NS045491/NS/NINDS NIH HHS/United States
- N01 MH032002/MH/NIMH NIH HHS/United States
- U01 MH083501/MH/NIMH NIH HHS/United States
- U01 MH083506/MH/NIMH NIH HHS/United States
- U01MH083545/MH/NIMH NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R21 DA026322/DA/NIDA NIH HHS/United States
- NS38841/NS/NINDS NIH HHS/United States
- U24 MH100929/MH/NIMH NIH HHS/United States
- U01 MH083507/MH/NIMH NIH HHS/United States
- U24 MH100931/MH/NIMH NIH HHS/United States
- R01 MH083588/MH/NIMH NIH HHS/United States
- 5U01MH083500/MH/NIMH NIH HHS/United States
- R24MH59724/MH/NIMH NIH HHS/United States
- R24 MH059745/MH/NIMH NIH HHS/United States
- R24MH59745/MH/NIMH NIH HHS/United States
- R24NS45491/NS/NINDS NIH HHS/United States
- MH083588/MH/NIMH NIH HHS/United States
- R24 NS038841/NS/NINDS NIH HHS/United States
- R25 MH080663/MH/NIMH NIH HHS/United States
- DA26322/DA/NIDA NIH HHS/United States
- R25MH080663/MH/NIMH NIH HHS/United States
- U01MH083501/MH/NIMH NIH HHS/United States
- U01MH083506/MH/NIMH NIH HHS/United States
- U01 MH083545/MH/NIMH NIH HHS/United States
- N01 MH022005/MH/NIMH NIH HHS/United States
- U01MH083507/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials