The evolution of the brain, the human nature of cortical circuits, and intellectual creativity - PubMed (original) (raw)
The evolution of the brain, the human nature of cortical circuits, and intellectual creativity
Javier Defelipe. Front Neuroanat. 2011.
Erratum in
- Front Neuroanat. 2013;7:10
Abstract
The tremendous expansion and the differentiation of the neocortex constitute two major events in the evolution of the mammalian brain. The increase in size and complexity of our brains opened the way to a spectacular development of cognitive and mental skills. This expansion during evolution facilitated the addition of microcircuits with a similar basic structure, which increased the complexity of the human brain and contributed to its uniqueness. However, fundamental differences even exist between distinct mammalian species. Here, we shall discuss the issue of our humanity from a neurobiological and historical perspective.
Keywords: brain size; cortical circuits; evolution; human nature; number synapses; pyramidal neurons.
Figures
Figure 1
The mind–body problem. Left: Fray Pedro de San Dionisio, painted by Francisco de Zurbarán (1598–1664). © Fundació Institut d'Art Hispanic Amatller. Arxiu Mas. Right: Don Quixote, Museum of Arte Moderno in Mexico. These images are examples of the separation between the mental and the physical worlds. The saint levitates while praying, and his head is separated from his body; Don Quixote appears reflective, with an empty head.
Figure 2
One small step for a man, one giant leap for mankind (Neil Armstrong). Left, photograph of the footprint left approximately 3.5 million years ago by A. afarensis, probably the immediate predecessor of the genus Homo, in Laetoli (Tanzania). Right, footprint in the lunar soil made by the astronaut Neil Armstrong (Apollo 11, July 21, 1969), the first person ever to set foot on the Moon. In a relatively short period of time we have gone from taking the first steps upright in Africa to walking on the Moon. How was this possible? While the brain has certainly increased in size during evolution it is not clear that this is the sole cause. Thus, the key question is whether the increase in the number of cortical circuits or rather changes in these circuits has been the driving force behind humans’ rapid development? The schemes of the size and shape of the brains (fossil endocranial casts) of the Australopithecus africanus, Homo erectus, and Homo sapiens were taken from Bermúdez de Castro (2002).
Figure 3
Table I from Spitzka (1907) which includes the name, age, occupation, nationality, and brain weight of different personalities.
Figure 4
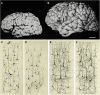
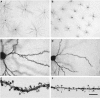
Increase in brain size and the maturation of cortical circuits. The maturation of mental processes and motor skills is associated with an approximately fourfold enlargement in brain size. (A,B) photographs of the brains of a 1-month and 6-year-old-child. This increment is accompanied by a dramatic development in the complexity of the neuronal processes, which in turn is influenced by the genetic background and the environment. This increase in the complexity is clearly evident in the drawings of Golgi stained cortical neurons from the cerebral cortex of a 1-month [(C) “pars triangularis of gyrus frontalis inferior”; (D) “orbital gyrus”] and 6 year [(E), “pars triangularis of gyrus frontalis inferior”; (F) “orbital gyrus”] old child. Adapted from Conel and Le (1941, 1967). Scale bar for (A,B): 2 cm.
Figure 5
Middle Stone Age engravings from South Africa. Photos and tracings of pieces M3-10, M2-1, and M1-6 from the Blombos Cave. In the drawing of the center is shown the stratigraphy and ages of Blombos Cave deposits. Adapted from Henshilwood et al. (2009).
Figure 6
Development of artistic creativity. Photographs of the Venus of Tan-Tan [6 cm in height, Morocco (A), the lionman of Hohlenstein–Stadel [29.6 cm, Germany (B), the Venus of Willendorf [11.1 cm, Austria (C), and David of Michelangelo [517 cm, created from a single block of marble between the years 1501 and 1504 (D). Taken from DeFelipe (2010a).
Figure 7
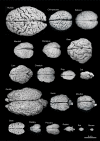
Variability of brain size and external topography. Photographs and weights of the brains of different species. Primates: human (Homo sapiens, 1.176 kg), chimpanzee (Pan troglodytes, 273 g), baboon (Papio cynocephalus, 151 g), mandrill (Mandrillus sphinx, 123 g), macaque (Macaca tonkeana, 110 g). Carnivores: bear (Ursus arctos, 289 g), lion (Panthera leo, 165 g), cheetah (Acinonyx jubatus, 119 g), dog (Canis familiaris, 95 g), cat (Felis catus, 32 g). Artiodactyls: giraffe (Giraffa camelopardalis, 700 g), kudu (Tragelaphus strepsiceros, 166 g), mouflon (Ovis musimon, 118 g), ibex (Capra pyrenaica, 115 g); peccary (Tayassu pecari, 41 g). Marsupials: wallaby (Protemnodon rufogrisea, 28 g). Lagomorphs: rabbit (Oryctolagus cuniculus, 5.2 g). Rodents: rat (Rattus rattus, 2.6 g), mouse (Mus musculus, 0.5 g). The chimpanzee brain was kindly supplied by Dr. Dean Falk. The rest of non-human brains were from material used in Ballesteros-Yánez et al., 2005). Scale bar: 5 cm.
Figure 8
Cytoarchitectonic differences in the mammalian cortex. Photomicrographs from 100 μm thick Nissl-stained sections showing some cytoarchitectonic differences between frontal, parietal, and occipital cortical areas of the human (areas 10, 3b, and 17, respectively) and several mammals. Scale bar: 250 μm. Adapted from Ballesteros-Yánez et al. (2005).
Figure 9
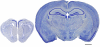
Evolutionary development of the cortex. Photomicrographs of coronal sections (100 μm thick Nissl-stained sections) of the telencephalon of a reptile (gecko, left) and a mammal (mouse, right), illustrating the evolutionary development of the cortex. Scale bar: 1000 μm.
Figure 10
Variation in brain size and patterns of convolutions. Coronal sections (25–40 μm thick; thionin staining) of the brain of several mammalian species. Adapted with permission from
, supported by the US National Science Foundation. Scale bar: 10 cm.
Figure 11
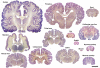
Variations in neuron density, cytoarchitectonic organization, and in the size and distribution of vertical neuron aggregates. (A–C) Low-power photomicrographs of 100-μm thick vibratome sections stained with thionin from (A) Brodmann's area 21 of the human, (B) the occipital cortex of the giraffe, and (C) the bill representation in the primary somatosensory cortex of the platypus. (D–F) High-power images showing some clear cytoarchitectonic differences between the three species: (D) presence of vertical aggregates of neurons or minicolumns (M) in layers II–III of the human area 39; (E) clusters of neurons (C) in layer II of the giraffe; (F) highly cellular dense layer II (La) in the platypus. Note the obvious differences in the density of cells between the three species. Scale bar in (F): 710 μm in (A–C); 100 μm in (D–F).
Figure 12
Electron microscopy and number of synapses per neuron. Left, Electron micrographs illustrating the neuropil of layer IIIa in the human temporal cortex, and of layer III of the mouse barrel cortex. Note the higher density of synapses (some of them indicated by arrows) in the mouse cortex. Scale bar = 0.5 μm. Right, Number of synapses per neuron in the human, rat, and mouse. The values obtained in layers II, IIIa, and IIIb of human, and layers Va and Vb of rat were recalculated according to the relative thickness of these layers to estimate the representative values of layers II–III and V, respectively. From DeFelipe et al. (2002).
Figure 13
Cortical microanatomical variations between humans, rats, and mice. Comparison of the thickness of layers, number of neurons and of the synaptic profiles within cubes of cortical tissue (50 μm wide by 50 μm thick) from the pial surface to the white matter in the human (anterolateral temporal cortex; T2–T3), rat (hindlimb area of the somatosensory cortex), and mouse (barrel cortex). L, cross-sectional length of synaptic junctions; AS, asymmetric synapses; SS, symmetric synapses. Values taken from DeFelipe et al. (2002).
Figure 14
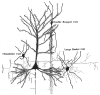
Pyramidal cells in the human and mouse neocortex. (A,B) Low-power photomicrographs of layer III pyramidal cells injected with Lucifer Yellow and processed with DAB in human (A) and mouse (B) temporal cortex. Note the smaller size of mouse cells. The section is parallel to the cortical surface. (C,D): Photomicrograph of horizontally projecting dendrites of a human (C) and mouse (D) pyramidal cell. (E,F): High-power photomicrographs of the basal dendritic segments of human (E) and mouse (F) pyramidal cells illustrating dendritic spines. Note the smaller size of the mouse spines. Scale bar in (F): 425 μm in (A,B); 45 μm in (C,D); 10 μm in (E,F). Taken from Benavides-Piccione et al. (2002).
Figure 15
Synaptic relationships between double bouquet cells, chandelier cells, and large basket cells with pyramidal cells. These cells constitute the best morphologically and chemically characterized types of interneurons. From DeFelipe and Fariñas (1992).
Figure 16
Microcolumnar organization of double bouquet cells. (A,B,D,E) Low magnification photomicrographs of calbindin immunostained (CB-ir) sections from the human: primary somatosensory (area 3b); temporal associative (area 22); and primary (area 17) and secondary (area 18) visual areas, showing the distribution of CB-ir cell bodies and double bouquet cell horse-tails. Note the large number and the regular distribution of double bouquet cell horse-tails. (C,F) High magnification of the boxed areas shown in (B,E), respectively, to highlight the differences in thickness, density, and number of axon collaterals of CB-ir double bouquet cell horse-tails in different cortical areas. Scale bar: 150 μm in (A,B,D,E); 45 μm in (C–F). Adapted from Ballesteros-Yánez et al. (2005).
Figure 17
Tyrosine hydroxylase interneurons. Low-power photomicrographs of tyrosine hydroxylase (TH) immunostaining in the temporal cortex of the human (anterolateral temporal lobe) (A), macaque (area TE) (B), somatosensory cortex of the gerbil (C), and somatosensory cortex of rat (D), extending from lower part of layer V to the white matter. Some TH neurons are indicated with arrows. TH neurons are relatively abundant in the deep layers of the human neocortex when compared to other species. However, they are occasionally found in the somatosensory cortex of the rat and gerbil whereas in the macaque monkey no TH neurons were observed in the in the lateral temporal cortex. Nevertheless, in other cortical areas of the monkey these cells are also present in deep layers. Scale bar = 160 μm.
Similar articles
- Microstructure of the neocortex: comparative aspects.
DeFelipe J, Alonso-Nanclares L, Arellano JI. DeFelipe J, et al. J Neurocytol. 2002 Mar-Jun;31(3-5):299-316. doi: 10.1023/a:1024130211265. J Neurocytol. 2002. PMID: 12815249 Review. - Not all brains are made the same: new views on brain scaling in evolution.
Herculano-Houzel S. Herculano-Houzel S. Brain Behav Evol. 2011;78(1):22-36. doi: 10.1159/000327318. Epub 2011 Jun 17. Brain Behav Evol. 2011. PMID: 21691045 Review. - Phylogenetic variation in cortical layer II immature neuron reservoir of mammals.
La Rosa C, Cavallo F, Pecora A, Chincarini M, Ala U, Faulkes CG, Nacher J, Cozzi B, Sherwood CC, Amrein I, Bonfanti L. La Rosa C, et al. Elife. 2020 Jul 21;9:e55456. doi: 10.7554/eLife.55456. Elife. 2020. PMID: 32690132 Free PMC article. - Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory.
Marín-Padilla M. Marín-Padilla M. J Comp Neurol. 1992 Jul 8;321(2):223-40. doi: 10.1002/cne.903210205. J Comp Neurol. 1992. PMID: 1500541 - [The evolution of the structure of the neocortex in mammals: a new theory of cytoarchitecture].
Marín Padilla M. Marín Padilla M. Rev Neurol. 2001 Nov 1-15;33(9):843-53. Rev Neurol. 2001. PMID: 11784988 Review. Spanish.
Cited by
- Structural changes of CA1 pyramidal neurons after stroke in the contralesional hippocampus.
Merino-Serrais P, Plaza-Alonso S, Hellal F, Valero-Freitag S, Kastanauskaite A, Plesnila N, DeFelipe J. Merino-Serrais P, et al. Brain Pathol. 2024 May;34(3):e13222. doi: 10.1111/bpa.13222. Epub 2023 Nov 27. Brain Pathol. 2024. PMID: 38012061 Free PMC article. - A biologically inspired repair mechanism for neuronal reconstructions with a focus on human dendrites.
Groden M, Moessinger HM, Schaffran B, DeFelipe J, Benavides-Piccione R, Cuntz H, Jedlicka P. Groden M, et al. PLoS Comput Biol. 2024 Feb 23;20(2):e1011267. doi: 10.1371/journal.pcbi.1011267. eCollection 2024 Feb. PLoS Comput Biol. 2024. PMID: 38394339 Free PMC article. - Modeling and Simulations in Time Domain of a Stimulation Set-up for Cortical Applications.
Schweigmann M, Kirchhoff F, Koch KP. Schweigmann M, et al. Eur J Transl Myol. 2016 Jun 13;26(2):6017. doi: 10.4081/ejtm.2016.6017. eCollection 2016 Jun 13. Eur J Transl Myol. 2016. PMID: 27478564 Free PMC article. - Amyloid Cascade Hypothesis for the Treatment of Alzheimer's Disease: Progress and Challenges.
Wu T, Lin D, Cheng Y, Jiang S, Riaz MW, Fu N, Mou C, Ye M, Zheng Y. Wu T, et al. Aging Dis. 2022 Dec 1;13(6):1745-1758. doi: 10.14336/AD.2022.0412. eCollection 2022 Dec 1. Aging Dis. 2022. PMID: 36465173 Free PMC article. - Variation and convergence in the morpho-functional properties of the mammalian neocortex.
Mahon S. Mahon S. Front Syst Neurosci. 2024 Jun 20;18:1413780. doi: 10.3389/fnsys.2024.1413780. eCollection 2024. Front Syst Neurosci. 2024. PMID: 38966330 Free PMC article. Review.
References
- Ballesteros-Yánez I., Muñoz A., Contreras J., Gonzalez J., Rodriguez-Veiga E., DeFelipe J. (2005). The double bouquet cell in the human cerebral cortex and a comparison with other mammals. J. Comp. Neurol. 486, 344–360 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources