Avian ultraviolet/violet cones identified as probable magnetoreceptors - PubMed (original) (raw)
Avian ultraviolet/violet cones identified as probable magnetoreceptors
Christine Niessner et al. PLoS One. 2011.
Abstract
Background: The Radical-Pair-Model postulates that the reception of magnetic compass directions in birds is based on spin-chemical reactions in specialized photopigments in the eye, with cryptochromes discussed as candidate molecules. But so far, the exact subcellular characterization of these molecules in the retina remained unknown.
Methodology/principal findings: We here describe the localization of cryptochrome 1a (Cry1a) in the retina of European robins, Erithacus rubecula, and domestic chickens, Gallus gallus, two species that have been shown to use the magnetic field for compass orientation. In both species, Cry1a is present exclusively in the ultraviolet/violet (UV/V) cones that are distributed across the entire retina. Electron microscopy shows Cry1a in ordered bands along the membrane discs of the outer segment, and cell fractionation reveals Cry1a in the membrane fraction, suggesting the possibility that Cry1a is anchored along membranes.
Conclusions/significance: We provide first structural evidence that Cry1a occurs within a sensory structure arranged in a way that fulfils essential requirements of the Radical-Pair-Model. Our findings, identifying the UV/V-cones as probable magnetoreceptors, support the assumption that Cry1a is indeed the receptor molecule mediating information on magnetic directions, and thus provide the Radical-Pair-Model with a profound histological background.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
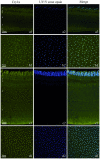
Figure 1. Immuno-labeling for Cry1a and UV cone opsin, and their co-localization in the retina.
(A), Vertical section of chicken retina; (B), whole mount of chicken retina; (C), vertical section of European robin retina; (D), whole mount of robin retina. The different layers in the vertical sections are indicated: 1 outer and inner segments of the photoreceptors with the oil droplets in between; 2 outer nuclear layer; 3 outer plexiform layer; 4 inner nuclear layer; 5 inner plexiform layer; 6 ganglion cell layer. Left column: (A1 to D1): Cry1a immunofluorescence (rendered in green) is inside the outer segment of a very slender photoreceptor type. Middle column: (A2 to D2): UV/V cone opsin immunofluorescence (rendered in blue) in the same section. Right column: (A3 to D3): Merge of the images, indicating that Cry1a and the UV/V cone opsin co-localize. In robins, the the population density of the Cry1a/UV appers to be higher than in chickens.
Figure 2. Electron-microscopic image and Western blots.
(A) Outer segment of a long, slender cone photoreceptor of the chicken retina, with the large oil droplet visible at the base and the connecting cilium (marked by the arrow) on the left. Cry1a, labeled with diaminobenzidin and silver intensification, is visible as dark dots along the disc membranes. (B) Higher magnification of the lower part of the outer segment in (A). Cry1a is found along some, but not all disks. At the left side with the connecting cilium, Cry1a is transported to the outer segment, where it is to bind to the membranes. (C) Outer segment of a cone photoreceptor of the robin retina, also showing ‘bands’ of Cry1a label. Western blot of robin (D) and of chicken retina (E), respectively. F1, cytosolic fraction; F2, membrane fraction; F3; nuclear fraction, F4, cytoskeletal fraction, T, tongue tissue from the same bird as control. Cry1a is found in the cytosolic and the membrane fraction in both species. Markers for the different fractions shown for chicken: (E1) Protein Kinase C for cytosolic, (E2) E-cadherin for membrane, (E3) Histon H3 for nuclear in chicken, (E4) Actin for cytoskeletal fractions (35). The markers show that F1 and F2 are free from other fractions (see Protein Kinase C and E-cadherin). E-cadherin is also bound to the actin cytoskeleton in F4, but a low ‘spill-over’ in F3 is visible. The same is true for Actin, the control for the cytoskeletal fractions. Histon H3 is in F3 but also in F4, because of its high abundance in the cell lysate.
Similar articles
- Magnetoreception: activated cryptochrome 1a concurs with magnetic orientation in birds.
Nießner C, Denzau S, Stapput K, Ahmad M, Peichl L, Wiltschko W, Wiltschko R. Nießner C, et al. J R Soc Interface. 2013 Aug 21;10(88):20130638. doi: 10.1098/rsif.2013.0638. Print 2013 Nov 6. J R Soc Interface. 2013. PMID: 23966619 Free PMC article. - Double-Cone Localization and Seasonal Expression Pattern Suggest a Role in Magnetoreception for European Robin Cryptochrome 4.
Günther A, Einwich A, Sjulstok E, Feederle R, Bolte P, Koch KW, Solov'yov IA, Mouritsen H. Günther A, et al. Curr Biol. 2018 Jan 22;28(2):211-223.e4. doi: 10.1016/j.cub.2017.12.003. Epub 2018 Jan 4. Curr Biol. 2018. PMID: 29307554 - Avian ultraviolet/violet cones as magnetoreceptors: The problem of separating visual and magnetic information.
Bischof HJ, Nießner C, Peichl L, Wiltschko R, Wiltschko W. Bischof HJ, et al. Commun Integr Biol. 2011 Nov 1;4(6):713-6. doi: 10.4161/cib.17338. Commun Integr Biol. 2011. PMID: 22446535 Free PMC article. - Sensing magnetic directions in birds: radical pair processes involving cryptochrome.
Wiltschko R, Wiltschko W. Wiltschko R, et al. Biosensors (Basel). 2014 Jul 24;4(3):221-42. doi: 10.3390/bios4030221. eCollection 2014 Sep. Biosensors (Basel). 2014. PMID: 25587420 Free PMC article. Review. - The Magnetic Compass of Birds: The Role of Cryptochrome.
Wiltschko R, Nießner C, Wiltschko W. Wiltschko R, et al. Front Physiol. 2021 May 19;12:667000. doi: 10.3389/fphys.2021.667000. eCollection 2021. Front Physiol. 2021. PMID: 34093230 Free PMC article. Review.
Cited by
- Cryptochrome expression in avian UV cones: revisiting the role of CRY1 as magnetoreceptor.
Pinzon-Rodriguez A, Muheim R. Pinzon-Rodriguez A, et al. Sci Rep. 2021 Jun 16;11(1):12683. doi: 10.1038/s41598-021-92056-8. Sci Rep. 2021. PMID: 34135416 Free PMC article. - The discovery of the use of magnetic navigational information.
Wiltschko R, Wiltschko W. Wiltschko R, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022 Jan;208(1):9-18. doi: 10.1007/s00359-021-01507-0. Epub 2021 Sep 2. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022. PMID: 34476571 Free PMC article. Review. - The chick eye in vision research: An excellent model for the study of ocular disease.
Wisely CE, Sayed JA, Tamez H, Zelinka C, Abdel-Rahman MH, Fischer AJ, Cebulla CM. Wisely CE, et al. Prog Retin Eye Res. 2017 Nov;61:72-97. doi: 10.1016/j.preteyeres.2017.06.004. Epub 2017 Jun 28. Prog Retin Eye Res. 2017. PMID: 28668352 Free PMC article. Review. - Magnetoreception: activation of avian cryptochrome 1a in various light conditions.
Nießner C, Denzau S, Peichl L, Wiltschko W, Wiltschko R. Nießner C, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018 Dec;204(12):977-984. doi: 10.1007/s00359-018-1296-7. Epub 2018 Oct 22. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018. PMID: 30350127 - Weak Broadband Electromagnetic Fields are More Disruptive to Magnetic Compass Orientation in a Night-Migratory Songbird (Erithacus rubecula) than Strong Narrow-Band Fields.
Schwarze S, Schneider NL, Reichl T, Dreyer D, Lefeldt N, Engels S, Baker N, Hore PJ, Mouritsen H. Schwarze S, et al. Front Behav Neurosci. 2016 Mar 22;10:55. doi: 10.3389/fnbeh.2016.00055. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27047356 Free PMC article.
References
- Wiltschko W. Über den Einfluß statischer Magnetfelder auf die Zugorientierung der Rotkehlchen, Erithacus rubecula. Z Tierpsychol. 1968;25:537–558. - PubMed
- Wiltschko W, Wiltschko R. Magnetoreception in birds: two receptors for two different tasks. J Ornithol. 2007;148,(Suppl. 1):S61–S76.
- Wiltschko W, Freire R, Munro U, Ritz T, Rogers L, et al. The magnetic compass of domestic chickens, Gallus gallus. J Exp Biol. 2007;210:2300–2310. - PubMed
- Wiltschko W, Traudt J, Güntürkün O, Prior H, Wiltschko R. Lateralization of magnetic compass orientation in a migratory bird. Nature. 2002;419:467–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases