Syntheses of 5-formyl- and 5-carboxyl-dC containing DNA oligos as potential oxidation products of 5-hydroxymethylcytosine in DNA - PubMed (original) (raw)
. 2011 Jul 1;13(13):3446-9.
doi: 10.1021/ol201189n. Epub 2011 Jun 7.
Affiliations
- PMID: 21648398
- PMCID: PMC3150843
- DOI: 10.1021/ol201189n
Syntheses of 5-formyl- and 5-carboxyl-dC containing DNA oligos as potential oxidation products of 5-hydroxymethylcytosine in DNA
Qing Dai et al. Org Lett. 2011.
Abstract
To investigate the potential oxidation products of 5-hydroxymethylcytosine (5-hmC)-containing DNA, we present here efficient syntheses of 5-formyl- and 5-methoxycarbonyl-2'-deoxycytidine phosphoramidites. The 5-formyl group in III was easy to introduce and was compatible with phosphoramidite and DNA syntheses. An additional treatment of ODN1 with NaBH(4) produced the corresponding ODN2 quantitatively. Phosphoramidite V was also incorporated into DNA, and the methyl ester could be hydrolyzed under mild basic conditions to afford ODN3.
Figures
Figure 1
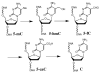
A possible demethylation pathway of 5-mC through 5-hmC, 5-fC, and 5-caC
Figure 2
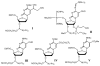
Structures of phosphoramidites for synthesis of DNA or RNA containing 5-fC or 5-caC modifications
Figure 3
(A) ODN1 was deprotected by treatment of 0.1 M K2CO3 in MeOH/H2O (1:1 v/v) at r.t. for 2 h. Peak a is the fully deprotected 5mer, [MH]+=1491. (B) ODN1 was deprotected by treatment of NH4OH at r.t. for 2 h, peak b is the proposed imine intermediate. (C) ODN1 obtained in the same way as (B) was dissoved in H2O and allowed to stand overnight at r.t., after which peak b disappeared. (D) ODN2 was deprotected by treatment of 0.1 M K2CO3 in MeOH/H2O (1:1 v/v) at r.t. for 2 h, peak c is the fully deprotected 5mer, [MH]+=1493. (E) ODN2 was deprotected by NH4OH at r.t. for 2 h. (F) ODN3 made from phoshoramidite IV was deprotected by treatment of 0.1 M K2CO3 in MeOH/H2O (1:1 v/v) at r.t. overnight. Peak d is the fully deprotected 5mer, [MH]+=1507; peak e is the 5mer with 5-trifluoroethoxycarbonyl-dC modification, [MH]+=1589. (G) ODN3 made from phoshoramidite V was deprotected by treatment of 0.1 M K2CO3 in MeOH/H2O (1:1 v/v) at r.t. overnight. (H) ODN3 made from phoshoramidite V was treated with NH4OH at r.t. for 2 h. Peak f is the 5mer with a 5-formamide modification, [MH]+=1506; peak g is the 5mer with a 5-methoxycarbonyl-dC modification, [MH]+ =1521.
Scheme 1
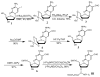
Synthesis of phosphoramidite III
Scheme 2
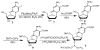
Synthesis of phosphoramidite V
Similar articles
- Syntheses of two 5-hydroxymethyl-2'-deoxycytidine phosphoramidites with TBDMS as the 5-hydroxymethyl protecting group and their incorporation into DNA.
Dai Q, Song CX, Pan T, He C. Dai Q, et al. J Org Chem. 2011 May 20;76(10):4182-8. doi: 10.1021/jo200566d. Epub 2011 Apr 14. J Org Chem. 2011. PMID: 21462947 Free PMC article. - 5-Hydroxymethylcytosine and 5-formylcytosine containing deoxyoligonucleotides: facile syntheses and melting temperature studies.
Xuan S, Wu Q, Cui L, Zhang D, Shao F. Xuan S, et al. Bioorg Med Chem Lett. 2015 Mar 15;25(6):1186-91. doi: 10.1016/j.bmcl.2015.01.070. Epub 2015 Feb 7. Bioorg Med Chem Lett. 2015. PMID: 25704892 - Improved synthesis and mutagenicity of oligonucleotides containing 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine.
Münzel M, Lischke U, Stathis D, Pfaffeneder T, Gnerlich FA, Deiml CA, Koch SC, Karaghiosoff K, Carell T. Münzel M, et al. Chemistry. 2011 Dec 2;17(49):13782-8. doi: 10.1002/chem.201102782. Epub 2011 Nov 8. Chemistry. 2011. PMID: 22069110 - Oxidized C5-methyl cytosine bases in DNA: 5-Hydroxymethylcytosine; 5-formylcytosine; and 5-carboxycytosine.
Klungland A, Robertson AB. Klungland A, et al. Free Radic Biol Med. 2017 Jun;107:62-68. doi: 10.1016/j.freeradbiomed.2016.11.038. Epub 2016 Nov 24. Free Radic Biol Med. 2017. PMID: 27890639 Review. - TET enzymatic oxidation of 5-methylcytosine, 5-hydroxymethylcytosine and 5-formylcytosine.
Cadet J, Wagner JR. Cadet J, et al. Mutat Res Genet Toxicol Environ Mutagen. 2014 Apr;764-765:18-35. doi: 10.1016/j.mrgentox.2013.09.001. Epub 2013 Sep 14. Mutat Res Genet Toxicol Environ Mutagen. 2014. PMID: 24045206 Review.
Cited by
- Chemical and enzymatic modifications of 5-methylcytosine at the intersection of DNA damage, repair, and epigenetic reprogramming.
Baljinnyam T, Sowers ML, Hsu CW, Conrad JW, Herring JL, Hackfeld LC, Sowers LC. Baljinnyam T, et al. PLoS One. 2022 Aug 29;17(8):e0273509. doi: 10.1371/journal.pone.0273509. eCollection 2022. PLoS One. 2022. PMID: 36037209 Free PMC article. - Retracted Article: Divergent synthesis of 5-substituted pyrimidine 2'-deoxynucleosides and their incorporation into oligodeoxynucleotides for the survey of uracil DNA glycosylases.
Tran A, Zheng S, White DS, Curry AM, Cen Y. Tran A, et al. Chem Sci. 2020 Oct 7;11(43):11818-11826. doi: 10.1039/d0sc04161k. Chem Sci. 2020. PMID: 34123208 Free PMC article. Retracted. - Neuronal enhancers are hotspots for DNA single-strand break repair.
Wu W, Hill SE, Nathan WJ, Paiano J, Callen E, Wang D, Shinoda K, van Wietmarschen N, Colón-Mercado JM, Zong D, De Pace R, Shih HY, Coon S, Parsadanian M, Pavani R, Hanzlikova H, Park S, Jung SK, McHugh PJ, Canela A, Chen C, Casellas R, Caldecott KW, Ward ME, Nussenzweig A. Wu W, et al. Nature. 2021 May;593(7859):440-444. doi: 10.1038/s41586-021-03468-5. Epub 2021 Mar 25. Nature. 2021. PMID: 33767446 Free PMC article. - Programmable site-selective labeling of oligonucleotides based on carbene catalysis.
Lee YH, Yu E, Park CM. Lee YH, et al. Nat Commun. 2021 Mar 16;12(1):1681. doi: 10.1038/s41467-021-21839-4. Nat Commun. 2021. PMID: 33727561 Free PMC article. - 5-Formylcytosine Yields DNA-Protein Cross-Links in Nucleosome Core Particles.
Li F, Zhang Y, Bai J, Greenberg MM, Xi Z, Zhou C. Li F, et al. J Am Chem Soc. 2017 Aug 9;139(31):10617-10620. doi: 10.1021/jacs.7b05495. Epub 2017 Jul 25. J Am Chem Soc. 2017. PMID: 28742335 Free PMC article.
References
- Kriaucionis S, Heintz N. Science. 2009;324:929. - PMC - PubMed
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Science. 2009;324:930. - PMC - PubMed
- Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Nature. 2010;466:1129. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM071440-07/GM/NIGMS NIH HHS/United States
- R01 GM088599/GM/NIGMS NIH HHS/United States
- R01 GM071440/GM/NIGMS NIH HHS/United States
- GM088599/GM/NIGMS NIH HHS/United States
- GM071440/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources