Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders - PubMed (original) (raw)
Review
Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders
Brian Giunta et al. Mol Brain. 2011.
Abstract
Up to 50% of long-term HIV infected patients, including those with systemically well-controlled infection, commonly experience memory problems and slowness, difficulties in concentration, planning, and multitasking. Deposition of Aβ plaques is also a common pathological feature of HIV infection. However, it is not clear whether this accumulation is due to AD-like processes, HIV-associated immunosuppression, Tat protein-induced Aβ elevations, and/or the effects of single highly active antiretroviral therapy (ART). Here we evaluated the effects of several ART medications (Zidovudine, Lamivudine, Indinavir, and Abacavir) alone and in combination on: 1) Aβ1-40, 42 generation in murine N2a cells transfected with the human "Swedish" mutant form of APP; 2) microglial phagocytosis of FITC-Aβ1-42 peptides in cultured murine N9 microglia. We report for the first time that these antiretroviral compounds (10 μM) generally increase Aβ generation (~50-200%) in SweAPP N2a cells and markedly inhibit microglial phagocytosis of FITC-Aβ1-42 peptides in murine microglia. The most significant amyloidogenic effects were observed with combined ART (p < 0.05); suggesting certain ART medications may have additive amyloidogenic effects when combined. As these antiretroviral compounds are capable of penetrating the blood brain barrier and reaching the concentrations employed in the in vitro studies, these findings raise the possibility that ART may play a casual role in the elevated Aβ found in the brains of those infected with HIV. Therefore these compounds may consequently contribute to cognitive decline observed in HIV associated neurocognitive disorders (HAND).
Figures
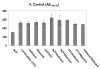
Figure 1
ART medications increase Aβ generation in cultured neuronal cells. Total Aβ 1-40,42 peptides were analyzed in conditioned media from SweAPP N2a cells by ELISA (n = 2 for each condition). Data are represented as a mean ± SEM percentage of Aβ 1-40,42 peptides secreted 18 hours after antiretroviral treatment relative to control (untreated). One-way ANOVA followed by post hoc comparison revealed significant increases in Aβ 1-40,42 production following 3TC/Indinavir, 3TC/Abacavir, and Indinavir/Abacavir treatements (concentration of 10 μM for each individual compound; p < 0.05).
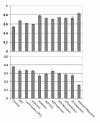
Figure 2
ART medications inhibit microglial phagocytosis of Aβ1-42 peptides. N9 microglia were treated with "aged" FITC-tagged Aβ 1-42 (300 nM) in complete medium for 120 min in the presence of 10 μM concentrations of antiretrovirals. Total FITC-Aβ1-42 peptides were analyzed by fluorescence from conditioned media (extracellular; top) and cell lysates (phagosomal/cell associated; bottom). Data are represented as the relative mean ± SEM fluorescence (n = 4 for each condition presented). When measuring extracellular FITC-tagged Aβ 1-42, one-way ANOVA followed by post hoc comparison showed significantly higher levels following all antiretroviral treatments, as compared to control (p < 0.05). When measuring cell associated FITC-tagged Aβ 1-42, one-way ANOVA followed by post hoc comparison showed significantly lower levels following Indinavir, Abacavir, AZT/3TC, AZT/Abacavir, 3TC/Indinavir, and Indinavir/Abacavir, as compared to control (p < 0.05).
Similar articles
- Efavirenz promotes β-secretase expression and increased Aβ1-40,42 via oxidative stress and reduced microglial phagocytosis: implications for HIV associated neurocognitive disorders (HAND).
Brown LA, Jin J, Ferrell D, Sadic E, Obregon D, Smith AJ, Tan J, Giunta B. Brown LA, et al. PLoS One. 2014 Apr 23;9(4):e95500. doi: 10.1371/journal.pone.0095500. eCollection 2014. PLoS One. 2014. PMID: 24759994 Free PMC article. - Does HIV infection contribute to increased beta-amyloid synthesis and plaque formation leading to neurodegeneration and Alzheimer's disease?
Fulop T, Witkowski JM, Larbi A, Khalil A, Herbein G, Frost EH. Fulop T, et al. J Neurovirol. 2019 Oct;25(5):634-647. doi: 10.1007/s13365-019-00732-3. Epub 2019 Mar 13. J Neurovirol. 2019. PMID: 30868421 Review. - Alterations in brain TREM2 and Amyloid-β levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy.
Fields JA, Spencer B, Swinton M, Qvale EM, Marquine MJ, Alexeeva A, Gough S, Soontornniyomkij B, Valera E, Masliah E, Achim CL, Desplats P. Fields JA, et al. J Neurochem. 2018 Dec;147(6):784-802. doi: 10.1111/jnc.14582. Epub 2018 Nov 26. J Neurochem. 2018. PMID: 30152135 Free PMC article. - Loss of cerebellar neurons in the progression of lentiviral disease: effects of CNS-permeant antiretroviral therapy.
Wächter C, Eiden LE, Naumann N, Depboylu C, Weihe E. Wächter C, et al. J Neuroinflammation. 2016 Oct 14;13(1):272. doi: 10.1186/s12974-016-0726-0. J Neuroinflammation. 2016. PMID: 27737697 Free PMC article. - Extracellular Vesicles: A Possible Link between HIV and Alzheimer's Disease-Like Pathology in HIV Subjects?
Kodidela S, Gerth K, Haque S, Gong Y, Ismael S, Singh A, Tauheed I, Kumar S. Kodidela S, et al. Cells. 2019 Aug 24;8(9):968. doi: 10.3390/cells8090968. Cells. 2019. PMID: 31450610 Free PMC article. Review.
Cited by
- Notch3 deletion regulates HIV-1 gene expression and systemic inflammation to ameliorate chronic kidney disease.
Thornton M, Sommer N, McGonigle M, Kumar Ram A, Yerrathota S, Ehirim H, Chaturvedi A, Dinh Phan J, Chakraborty A, Chakravarthi VP, Gunewardena S, Tyagi M, Talreja J, Wang T, Singhal P, Tran PV, Fields TA, Ray PE, Dhillon NK, Sharma M. Thornton M, et al. Dis Model Mech. 2025 Feb 1;18(2):DMM052056. doi: 10.1242/dmm.052056. Epub 2025 Feb 25. Dis Model Mech. 2025. PMID: 39910908 Free PMC article. - Independent effects of HIV, aging, and HAART on brain volumetric measures.
Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Ances BM, et al. J Acquir Immune Defic Syndr. 2012 Apr 15;59(5):469-77. doi: 10.1097/QAI.0b013e318249db17. J Acquir Immune Defic Syndr. 2012. PMID: 22269799 Free PMC article. - HIV and Alzheimer's disease: complex interactions of HIV-Tat with amyloid β peptide and Tau protein.
Hategan A, Masliah E, Nath A. Hategan A, et al. J Neurovirol. 2019 Oct;25(5):648-660. doi: 10.1007/s13365-019-00736-z. Epub 2019 Apr 23. J Neurovirol. 2019. PMID: 31016584 Free PMC article. Review. - HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS.
Cisneros IE, Ghorpade A. Cisneros IE, et al. Curr HIV Res. 2012 Jul;10(5):392-406. doi: 10.2174/157016212802138832. Curr HIV Res. 2012. PMID: 22591363 Free PMC article. Review. - Risk Factors and Pathogenesis of HIV-Associated Neurocognitive Disorder: The Role of Host Genetics.
Olivier IS, Cacabelos R, Naidoo V. Olivier IS, et al. Int J Mol Sci. 2018 Nov 14;19(11):3594. doi: 10.3390/ijms19113594. Int J Mol Sci. 2018. PMID: 30441796 Free PMC article. Review.
References
- Goodkin K, Fletcher MA, Cohen N. Clinical aspects of psychoneuroimmunology. Lancet. 1995;345(8943):183–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials