Distinct functional constraints partition sequence conservation in a cis-regulatory element - PubMed (original) (raw)
Distinct functional constraints partition sequence conservation in a cis-regulatory element
Antoine Barrière et al. PLoS Genet. 2011 Jun.
Abstract
Different functional constraints contribute to different evolutionary rates across genomes. To understand why some sequences evolve faster than others in a single cis-regulatory locus, we investigated function and evolutionary dynamics of the promoter of the Caenorhabditis elegans unc-47 gene. We found that this promoter consists of two distinct domains. The proximal promoter is conserved and is largely sufficient to direct appropriate spatial expression. The distal promoter displays little if any conservation between several closely related nematodes. Despite this divergence, sequences from all species confer robustness of expression, arguing that this function does not require substantial sequence conservation. We showed that even unrelated sequences have the ability to promote robust expression. A prominent feature shared by all of these robustness-promoting sequences is an AT-enriched nucleotide composition consistent with nucleosome depletion. Because general sequence composition can be maintained despite sequence turnover, our results explain how different functional constraints can lead to vastly disparate rates of sequence divergence within a promoter.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
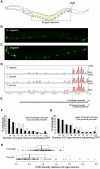
Figure 1. Conserved portions of unc-47 promoters direct spatially correct, but not robust, expression.
(A) A schematic depiction of GABAergic neurons in C. elegans. (B) Expression pattern of C. elegans promoter unc-47::GFP in C. elegans. (C) Expression pattern of C. briggsae promoter unc-47::GFP in C. briggsae. Photographs of worms are composites of multiple exposures of the same individual that capture the full complement of D-type neurons, in all focal planes, expressing GFP. (D) Vista plot of primary sequence conservation in the promoter region of unc-47 from C. briggsae, C. remanei and C. brenneri aligned to C. elegans. Window size = 20 bp, threshold = 70%. (E) Schematic depiction of full-length and proximal promoters. Consistency of GFP expression in D-type neurons for full-length and proximal promoters from C. elegans (F) and C. briggsae (G). For both C. elegans and C. briggsae, the average number of cells expressing GFP is lower for the proximal promoter compared to full-length (Wilcoxon test, p = 3.2×10−3 and p = 3.4×10−8, respectively). (H) Distribution of ratios of GFP expression intensity in DVB relative to D-type neurons for the full-length and proximal promoters at 20°C. Each strain is represented by 100 animals. The two distributions are significantly different (Wilcoxon test, p = 1.8×10−5; Kolmogorov-Smironov test, p = 1.1×10−8).
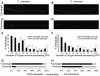
Figure 2. Promoters of C. brenneri and C. remanei unc-47 require the distal sequences for consistent expression.
GFP expression driven by full-length C. brenneri (A) and C. remanei (B) and proximal C. brenneri (C) and C. remanei (D) unc-47 promoters in a C. elegans host. As in Figure 1F, 1G, 200 individuals bearing each transgene were counted, and the percentages of those individuals with the indicated number of D-type neurons expressing GFP is shown (E–F). (G–H) Presence/absence of GFP expression in the cell DVB in the same 200 individuals for each of the four promoters. Photographs of worms are composites of multiple exposures of the same individual that capture the full complement of D-type neurons, in all focal planes, expressing GFP.
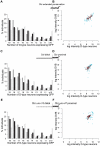
Figure 3. Only full-length promoters of C. elegans and C. briggsae unc-47 can direct robust expression.
Distribution of fluorescence intensity driven by C. elegans full-length (A) and proximal (B), and C. briggsae full-length (C) and proximal (D) promoters in C. elegans at two temperatures (red for 26°C and blue for 15°C). For each individual, the log intensity in D-type neurons is plotted against the log intensity in DVB. Individuals that did not show any fluorescence in DVB were excluded from analysis. Data for additional strains are given in Figure S4. Superimposed on each graph is a schematic of the construct used: a straight line represents C. elegans promoter of unc-47, a wavy line represents C. briggsae promoter of unc-47. The gray vertical bar indicates the 5′ boundary of the proximal promoter.
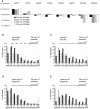
Figure 4. Different components of the distal promoter sequences regulate consistent expression and expression levels under temperature stress.
(A) Percentage of 200 individuals expressing GFP in the indicated number of D-type neurons under control of C. briggsae promoter with extended conservation, shown in solid black bars compared to C. briggsae full-length (black hashed bars) and proximal (gray hashed bars) promoters. (B) Intensity of GFP expression in D-type neurons and the cell DVB for animals bearing the extended conservation promoter reared at 26°C (red) or 15°C (blue). (C) Percentage of 200 individuals expressing GFP in the indicated number of D-type neurons under control of chimeric promoter fusion of C. elegans distal unc-47 promoter sequence and C. briggsae proximal promoter. For comparison, distributions for C. briggsae full-length and proximal promoters are shown in black and gray hashes, respectively. (D) Intensity of GFP in D-type neurons and the cell DVB for animals bearing the chimeric promoter reared at 26°C (red) or 15°C (blue). The chimeric promoter drives robust expression under temperature stress. (E) Percentage of 200 individuals expressing GFP in indicated number of D-type neurons from a chimeric promoter composed of distal C. briggsae unc-15 sequence and the C. briggsae unc-47 proximal promoter (black bars). For comparison, C. briggsae unc-47 full-length and proximal promoters are shown in black and gray hashed bars, respectively. The unc-15/unc-47 chimera is indistinguishable from the C. briggsae full-length promoter (Wilcoxon test p = 0.37), and it is significantly more consistent than the proximal promoter (Wilcoxon test p = 1.3×10−5). (F) Robustness of unc-15/unc-47 chimeric promoter under temperature stress.
Figure 5. Promoter regions that confer robustness are enriched for nucleosome-depleted sequences.
(A) Enrichment/depletion of dinucleotides in the distal promoters of unc-15, unc-25 and unc-47 genes relative to the sequence of the pPD95.75 vector (log scale). Percentage of 200 individuals expressing GFP in indicated number of D-type neurons from a chimeric promoter composed of AT-rich sequence from the C. elegans daf-25 locus (B), AT-poor sequence from the C. elegans let-2 locus (C), AT-rich sequence from the D. melanogaster ChAT locus (D), and AT-poor sequence from the D. melanogaster CG8394.2 locus (E), fused upstream of the C. briggsae unc-47 proximal promoter. For comparison, C. briggsae unc-47 full-length and proximal promoters are shown in black and gray hashed bars, respectively.
Similar articles
- Coevolution within and between regulatory loci can preserve promoter function despite evolutionary rate acceleration.
Barrière A, Gordon KL, Ruvinsky I. Barrière A, et al. PLoS Genet. 2012 Sep;8(9):e1002961. doi: 10.1371/journal.pgen.1002961. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028368 Free PMC article. - Conservation of function and expression of unc-119 from two Caenorhabditis species despite divergence of non-coding DNA.
Maduro M, Pilgrim D. Maduro M, et al. Gene. 1996 Dec 12;183(1-2):77-85. doi: 10.1016/s0378-1119(96)00491-x. Gene. 1996. PMID: 8996090 - Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence.
Gordon KL, Arthur RK, Ruvinsky I. Gordon KL, et al. PLoS Genet. 2015 May 28;11(5):e1005268. doi: 10.1371/journal.pgen.1005268. eCollection 2015 May. PLoS Genet. 2015. PMID: 26020930 Free PMC article. - Evolution of cis-regulatory sequence and function in Diptera.
Wittkopp PJ. Wittkopp PJ. Heredity (Edinb). 2006 Sep;97(3):139-47. doi: 10.1038/sj.hdy.6800869. Epub 2006 Jul 19. Heredity (Edinb). 2006. PMID: 16850038 Review. - Tempo and mode in evolution of transcriptional regulation.
Gordon KL, Ruvinsky I. Gordon KL, et al. PLoS Genet. 2012 Jan;8(1):e1002432. doi: 10.1371/journal.pgen.1002432. Epub 2012 Jan 19. PLoS Genet. 2012. PMID: 22291600 Free PMC article. Review.
Cited by
- Evolutionary meandering of intermolecular interactions along the drift barrier.
Lynch M, Hagner K. Lynch M, et al. Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):E30-8. doi: 10.1073/pnas.1421641112. Epub 2014 Dec 22. Proc Natl Acad Sci U S A. 2015. PMID: 25535374 Free PMC article. - Macro-level modeling of the response of C. elegans reproduction to chronic heat stress.
McMullen PD, Aprison EZ, Winter PB, Amaral LA, Morimoto RI, Ruvinsky I. McMullen PD, et al. PLoS Comput Biol. 2012 Jan;8(1):e1002338. doi: 10.1371/journal.pcbi.1002338. Epub 2012 Jan 26. PLoS Comput Biol. 2012. PMID: 22291584 Free PMC article. - Coevolution within and between regulatory loci can preserve promoter function despite evolutionary rate acceleration.
Barrière A, Gordon KL, Ruvinsky I. Barrière A, et al. PLoS Genet. 2012 Sep;8(9):e1002961. doi: 10.1371/journal.pgen.1002961. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028368 Free PMC article. - Shadow Enhancers Are Pervasive Features of Developmental Regulatory Networks.
Cannavò E, Khoueiry P, Garfield DA, Geeleher P, Zichner T, Gustafson EH, Ciglar L, Korbel JO, Furlong EE. Cannavò E, et al. Curr Biol. 2016 Jan 11;26(1):38-51. doi: 10.1016/j.cub.2015.11.034. Epub 2015 Dec 10. Curr Biol. 2016. PMID: 26687625 Free PMC article. - Shadow enhancers: frequently asked questions about distributed cis-regulatory information and enhancer redundancy.
Barolo S. Barolo S. Bioessays. 2012 Feb;34(2):135-41. doi: 10.1002/bies.201100121. Epub 2011 Nov 15. Bioessays. 2012. PMID: 22083793 Free PMC article.
References
- Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, et al. Ultraconserved elements in the human genome. Science. 2004;304(5675):1321–1325. - PubMed
- Boffelli D, Nobrega MA, Rubin EM. Comparative genomics at the vertebrate extremes. Nature Reviews Genetics. 2004;5(6):456–465. - PubMed
- Pennacchio LA, Rubin EM. Genomic strategies to identify mammalian regulatory sequences. Nature Reviews Genetics. 2001;2(2):100–109. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources