Myocilin, a glaucoma-associated protein, promotes cell migration through activation of integrin-focal adhesion kinase-serine/threonine kinase signaling pathway - PubMed (original) (raw)
Myocilin, a glaucoma-associated protein, promotes cell migration through activation of integrin-focal adhesion kinase-serine/threonine kinase signaling pathway
Heung Sun Kwon et al. J Cell Physiol. 2011 Dec.
Abstract
The MYOCILIN gene encodes a secreted glycoprotein which is highly expressed in eye drainage structures. Mutations in this gene may lead to juvenile open-angle glaucoma and adult onset primary open-angle glaucoma, one of the leading causes of irreversible blindness in the world. Functions of wild-type myocilin are still unclear. We have recently demonstrated that myocilin is a modulator of Wnt signaling and may affect actin cytoskeleton organization. Here we report that myocilin and its naturally occurring proteolytic fragments, similar to Wnt3a, are able to stimulate trabecular meshwork, NIH3T3, and FHL124 cell migration with the N-terminal proteolytic fragment of myocilin lacking the olfactomedin domain producing the highest stimulatory effect. Stimulation of cell migration occurs through activation of the integrin-focal adhesion kinase (FAK)-serine/threonine kinase (AKT) signaling pathway. Inhibition of FAK by siRNA reduced the stimulatory action of myocilin by threefold. Activation of several components of this signaling pathway was also demonstrated in the eyes of transgenic mice expressing elevated levels of myocilin in the eye drainage structures. These data extend the similarities between actions of myocilin and Wnt proteins acting through a β-catenin-independent mechanism. The modification of the migratory ability of cells by myocilin may play a role in normal functioning of the eye anterior segment and its pathology including glaucoma.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
Figure 1
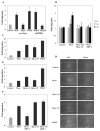
Effects of myocilin on cell migration. (A) Transwell assay. NIH3T3 cells were plated in the upper chamber while myocilin (3 μg/ml) or Wnt3a (3 μg/ml) diluted in PBS were added to the lower chamber. PBS was used as control. The cells migrating to the bottom surface of the filter after 6 hrs were trypsinized and counted using a hematocytometer. In some cases, myocilin and Wnt3a were preincubated with antibodies against mouse myocilin (1:1000 dilution) for 30 min before addition to the lower chamber. Changes in the amounts of migrating cells after addition of indicated proteins of antibodies were calculated relative to PBS control. (B) Scratch assay. Subconfluent monolayers of human TM cells were plated on glass chamber slides. The monolayers were scratched with a pipette tip and incubated for 3 or 6 hrs with purified myocilin (3 μg/ml). Serum-free medium was used as control. In some cases, myocilin was preincubated with antibodies against mouse myocilin or purified sFRP1. Cell migration was evaluated using Axiovision Rel. 4.7 software. (C) Transwell assay as in A but CM from HEK293 cells expressing myocilin or its proteolytic fragments was added to the lower chamber instead of purified myocilin. Conditioned medium from HEK293 cells transfected with vector was used as control. PDGF (10 ng/ml) was used as a positive control for cell migration. (D) Scratch assay. Scale bar, 20 μm. Subconfluent monolayers of NIH3T3 cells were plated on non-coated glass chamber slides. The monolayers were scratched with a pipette tip and incubated for 24 hrs with CM from HEK293 transiently transfected with myocilin, its proteolytic fragments, or vector (control). PDGF (10 ng/ml) was used as positive control. Cell migration was evaluated as in (B). (E) Quantification of the results shown in (D). Comparison between all samples gave statistically significant differences (p < 0.002). (F) NIH3T3 cells were grown as in (D). CM from HEK292 cells transiently transfected with vector (control), myocilin or myocilin-ΔN was preincubated with 10 μg/ml of sFRP1 for 30 min before addition to cells. Cell migration was evaluated as above. Quantification of the results is shown.
Figure 2
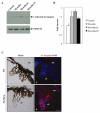
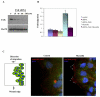
Effects of myocilin on β1-integrin levels in vitro and in vivo. (A) FHL124 cells were treated with CM from cells expressing myocilin or its proteolytic fragments for 6 hrs. Conditioned medium from HEK293 cells transfected with vector was used as control. The levels of β1-integrin in cell lysates were estimated by western blotting. (B) Quantification of the results shown in (A). (C) Frozen eye sections from control (a, b) and transgenic mice expressing elevated levels of wild-type myocilin in the eye drainage structures (c, d) were stained with antibodies against β1-integrin. (a and c) represent phase contrast, b and d) show immunofluorescence. Scale bar, 20 μm; cb - ciliary body; tm – trabecular meshwork.
Figure 3
Effects of myocilin on α-integrin and fibronectin levels in vitro. NIH3T3 cells were treated with CM from HEK293 cells expressing myocilin for 3 hrs. The levels of fibronectin and integrins were estimated after immunostaining of cells (A) or in cell lysates by western blotting (B). (C) Quantification of the results shown in (B). Scale bar in A, 10 μm.
Figure 4
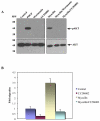
Myocilin stimulates FAK both in vitro and in vivo. FHL124 cells were treated for 3 hrs with CM from HEK293 cells expressing myocilin or its proteolytic fragments. PDGF (10 ng/ml) was used as a positive control. Shown are western blots of cell lysates stained with antibodies against activated FAK (A). (B) Quantification of the results shown in (A). Comparison between different samples with one exception gave statistically significant differences (p < 0.002). Difference between the mean values obtained for Myocilin-ΔC and Myocilin-ΔN was statistically insignificant (p = 0.37). Upregulation of activated FAK in the eyes of 20-month-old wild-type and transgenic mice (C). Frozen sections of wild type (a, c) and transgenic (b, d) eyes were stained with antibodies to phosphor FAK (1:200 dilution) and DAPI. 3 pairs of animals were analyzed. A typical staining pattern is shown. cb, ciliary body; tm, trabecular meshwork. Scale bar, 20 μm.
Figure 5
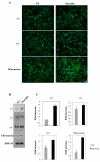
FAK is essential for myocilin-induced cell migration. (A) Inhibition of FAK in HEK293 cells transfected by increasing concentrations (0–100 nM) of FAK siRNAs. Shown are western blots of cell lysates stained with anti FAK antibodies 48 hrs after transfection. (B) Inhibition of myocilin-induced FHL124 cell migration by FAK siRNAs as judged by wound healing assay. Cells were mock transfected (control), transfected with scrambled siRNA or transfected with 100 nM FAK siRNAs and myocilin. Changes in cell migration between control, scrambled siRNA, siRNA-FAK and siRNA-FAK + myocilin were statistically insignificant (p between 0.06 and 0.33). (C) Accumulation of activated FAK at the leading edge of FHL124 migrating cells in wound healing assay. FHL124 cell monolayers were scratched and treated with CM from HEK293 cells expressing myocilin or CM from mocked transfected HEK293 cells (control) for 3 hrs, and stained with antibodies against phosphorylated FAK.
Figure 6
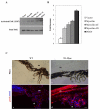
Myocilin induces Akt activity. (A) Treatment of FHL124 cells with myocilin (3 μg/ml) or PDGF (10 ng/ml) for 1 h led to activation of Akt. PBS was added to control samples. Inhibitors of PI3K, wortmannin (10 μM) and Ly294002 (20 μM), completely blocked the Akt activation. (B) Inhibition of myocilin-induced migration of FHL124 cells in would healing assay by LY294002 (20 μM). The monolayer of FHL124 cells was scratched with a pipette tip and incubated for 24 hrs with purified myocilin (3μg/ml) and LY294002 (20 μM). Control was incubated with PBS. Comparison between different samples with one exception gave statistically significant differences (p < 0.017). Difference between the mean values obtained for control and myocilin/LY294002 was statistically insignificant (p = 0.42).
Figure 7
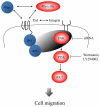
Schematic diagram of myocilin signaling in cell migration. Binding of myocilin or Wnt to frizzled receptors may enhance the association of Dvl with APC leading to the activation of FAK and its accumulation at the leading edge. Downstream targets of activated FAK include PI3K lipid kinase and Akt serine/threonine kinase. Inhibition of these kinases reduced cell migration. Myocilin proteolytic fragments also stimulate cell migration through the same mechanism.
Similar articles
- Wnt activation by wild type and mutant myocilin in cultured human trabecular meshwork cells.
Shen X, Ying H, Yue BY. Shen X, et al. PLoS One. 2012;7(9):e44902. doi: 10.1371/journal.pone.0044902. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028669 Free PMC article. - Myocilin is a modulator of Wnt signaling.
Kwon HS, Lee HS, Ji Y, Rubin JS, Tomarev SI. Kwon HS, et al. Mol Cell Biol. 2009 Apr;29(8):2139-54. doi: 10.1128/MCB.01274-08. Epub 2009 Feb 2. Mol Cell Biol. 2009. PMID: 19188438 Free PMC article. - Myocilin regulates cell proliferation and survival.
Joe MK, Kwon HS, Cojocaru R, Tomarev SI. Joe MK, et al. J Biol Chem. 2014 Apr 4;289(14):10155-67. doi: 10.1074/jbc.M113.547091. Epub 2014 Feb 22. J Biol Chem. 2014. PMID: 24563482 Free PMC article. - Myocilin and glaucoma: facts and ideas.
Tamm ER. Tamm ER. Prog Retin Eye Res. 2002 Jul;21(4):395-428. doi: 10.1016/s1350-9462(02)00010-1. Prog Retin Eye Res. 2002. PMID: 12150989 Review. - The effects of myocilin expression on functionally relevant trabecular meshwork genes: a mini-review.
Borrás T. Borrás T. J Ocul Pharmacol Ther. 2014 Mar-Apr;30(2-3):202-12. doi: 10.1089/jop.2013.0218. Epub 2014 Feb 24. J Ocul Pharmacol Ther. 2014. PMID: 24564495 Free PMC article. Review.
Cited by
- RNA Sequencing Reveals the Activation of Wnt Signaling in Low Flow Rate Brain Arteriovenous Malformations.
Huo R, Fu W, Li H, Jiao Y, Yan Z, Wang L, Wang J, Wang S, Cao Y, Zhao J. Huo R, et al. J Am Heart Assoc. 2019 Jun 18;8(12):e012746. doi: 10.1161/JAHA.119.012746. Epub 2019 Jun 7. J Am Heart Assoc. 2019. PMID: 31170876 Free PMC article. - Effects of transforming growth factor-β2 on myocilin expression and secretion in human primary cultured trabecular meshwork cells.
Wu Y, Chen W, Guo M, He Q, Hu Y. Wu Y, et al. Int J Clin Exp Pathol. 2014 Jul 15;7(8):4827-36. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25197353 Free PMC article. - Proteomic characterization of the normal human medial meniscus body using data-independent acquisition mass spectrometry.
Folkesson E, Turkiewicz A, Rydén M, Hughes HV, Ali N, Tjörnstrand J, Önnerfjord P, Englund M. Folkesson E, et al. J Orthop Res. 2020 Aug;38(8):1735-1745. doi: 10.1002/jor.24602. Epub 2020 Jan 31. J Orthop Res. 2020. PMID: 31989678 Free PMC article. - TRPV4-Rho signaling drives cytoskeletal and focal adhesion remodeling in trabecular meshwork cells.
Lakk M, Križaj D. Lakk M, et al. Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C1013-C1030. doi: 10.1152/ajpcell.00599.2020. Epub 2021 Mar 31. Am J Physiol Cell Physiol. 2021. PMID: 33788628 Free PMC article. - Pathogenesis of glaucoma: Extracellular matrix dysfunction in the trabecular meshwork-A review.
Keller KE, Peters DM. Keller KE, et al. Clin Exp Ophthalmol. 2022 Mar;50(2):163-182. doi: 10.1111/ceo.14027. Epub 2022 Jan 17. Clin Exp Ophthalmol. 2022. PMID: 35037377 Free PMC article. Review.
References
- Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Human Molec Genetics. 1997;6:2091–2097. - PubMed
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) New England J Med. 1998;338:1022–1027. - PubMed
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. - PubMed
- Aroca-Aguilar JD, Sanchez-Sanchez F, Ghosh S, Coca-Prados M, Escribano J. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J Biol Chem. 2005;280:21043–21051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous