Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory - PubMed (original) (raw)
Review
Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory
Stephen Maren. Neuron. 2011.
Abstract
Learning to contend with threats in the environment is essential to survival, but dysregulation of memories for traumatic events can lead to disabling psychopathology. Recent years have witnessed an impressive growth in our understanding of the neural systems and synaptic mechanisms underlying emotional memory formation. As a consequence, interest has emerged in developing strategies for suppressing, if not eliminating, fear memories. Here, I review recent work employing sophisticated behavioral, pharmacological, and molecular tools to target fear memories, placing these memories firmly behind the crosshairs of neurobiologically informed interventions.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
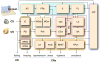
Figure 1
Neuronatomy of conditioned fear. A simplified neuroanatomical schematic outlining some of the major brain regions and their anatomical connections invoved in Pavlovian fear conditioning. Shading indicates major brain regions (brown, midbrain; yellow, thalamus; orange, basal forebrain; blue, neocortex; red, amygdala; violet, hippocampus). Sensory information asends to the amygdala through the midbrain and thalamus; auditory information also reaches the amygdala via the cortex. Antomical convergence and association of of the conditioned stimulus (CS) and unconditioned stimulus (US) occurs in the amygdala; contextual information processed by the hippocampus can also enter into association with the US in the amygdala. Conditioned and unconditioned fear responses (CRs and URs) are mediated by projections from the amygdala to an array of brain areas involved in autonomic and somatic defensive responses. Abbreviations: AC, auditory cortex; BA, basal nuclei of the amygdala; BNST, bed nuclei of the stria terminalis; CEl, lateral division of central nucleus of the amygdala; CEm, medial divsion of the central nucleus of the amygdala; HIP, hippocampus; IC, inferior colliculus; IL, infralimbic division of the medial prefrontal cortex; ITC, intercalated cellsof the amygdala; LA, lateral nucleus of the amygdala; LH, lateral hypothalamus; MGdv, dorsal and ventral divisions of the thalamic medial geniculate nucleus; MGm, medial division of the thalamic medial geniculate nucleus; NAcc, nucleus accumbens, dlPAG, dorsolateral division of the periaqueductal gray; vPAG, ventral division of the periaquedcutal gray; PIN, posterior intralaminar nucleus of the thalamus; PL, prelimbic division of the medial prefrontal cortex; PRh, perirhinal cortex; PVN, paraventricular nucleus of the hypothalamus.
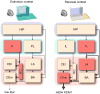
Figure 2
Contextual control of extinction. A simplfied neuroantaomical schematic illustrating differential c-fos expression in brain structures involved in the expression of fear after extinction. Suppression of fear to a CS in the extinction context (left) is associated with activity in the infralimbic division of the medial prefrontal cortex (IL) and inhibitory intercalated neurons (ITC) in the amygdala. Inhibition of the medial division of the central nucleus (CEm) by the ITC limits the expression of conditioned fear. The return of fear to a CS presented outside of the extinction context (Renewal context, right) is associated with activity in the prelimbic division of the medial prefrontal cortex (PL), the lateral nucleus of the amygdala (LA), and CEm. The hippocampus (HIP) basal nuclei of the amygdala (BA) are engaged in both situations, and may therefore gate the expression of conditioned fear through their connections with the the unique prefrontal-amygdala networks associated with extinction and renewal.
Figure 3
Stabilization and destabilization of memory. Memory formation is associated with initial encoding to establish a short-term memory (STM) followed by a time-dependent consolidation phase to establish a stable long-term memory (LTM). Retrieval of LTM destabilizes or deconsolidates (decon.) the memory (LTMr) rendering it labile once more; the persistence of LTM after retrieval requires reconsolidation (recon.). Failure to reconsolidate the memory trace results in decay, much as STM decays in the absence of consolidation. In the absence of retrieval, LTM may be actively erased by a variety of manipulations by interfering with the molecular mechanisms involved in memory maintenance.
Similar articles
- Erasing fear memories with extinction training.
Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Quirk GJ, et al. J Neurosci. 2010 Nov 10;30(45):14993-7. doi: 10.1523/JNEUROSCI.4268-10.2010. J Neurosci. 2010. PMID: 21068303 Free PMC article. Review. - Neural and cellular mechanisms of fear and extinction memory formation.
Orsini CA, Maren S. Orsini CA, et al. Neurosci Biobehav Rev. 2012 Aug;36(7):1773-802. doi: 10.1016/j.neubiorev.2011.12.014. Epub 2012 Jan 2. Neurosci Biobehav Rev. 2012. PMID: 22230704 Free PMC article. Review. - Modulation of fear memory by retrieval and extinction: a clue for memory deconsolidation.
Hong I, Kim J, Song B, Park S, Lee J, Kim J, An B, Lee S, Choi S. Hong I, et al. Rev Neurosci. 2011;22(2):205-29. doi: 10.1515/RNS.2011.023. Rev Neurosci. 2011. PMID: 21476941 Review. - Prefrontal dopamine D4 receptors are involved in encoding fear extinction.
Pfeiffer UJ, Fendt M. Pfeiffer UJ, et al. Neuroreport. 2006 May 29;17(8):847-50. doi: 10.1097/01.wnr.0000220142.29413.6f. Neuroreport. 2006. PMID: 16708027 - Neuronal circuits of fear extinction.
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Herry C, et al. Eur J Neurosci. 2010 Feb;31(4):599-612. doi: 10.1111/j.1460-9568.2010.07101.x. Epub 2010 Feb 11. Eur J Neurosci. 2010. PMID: 20384807 Review.
Cited by
- Social buffering diminishes fear response but does not equal improved fear extinction.
Gorkiewicz T, Danielewski K, Andraka K, Kondrakiewicz K, Meyza K, Kaminski J, Knapska E. Gorkiewicz T, et al. Cereb Cortex. 2023 Apr 4;33(8):5007-5024. doi: 10.1093/cercor/bhac395. Cereb Cortex. 2023. PMID: 36218820 Free PMC article. - Dopaminergic D2-like receptor stimulation affects attention on contextual information and modulates BOLD activation of extinction-related brain areas.
Nostadt A, Nitsche MA, Tegenthoff M, Lissek S. Nostadt A, et al. Sci Rep. 2023 Nov 28;13(1):21003. doi: 10.1038/s41598-023-47704-6. Sci Rep. 2023. PMID: 38017050 Free PMC article. - Unrelenting Fear Under Stress: Neural Circuits and Mechanisms for the Immediate Extinction Deficit.
Maren S. Maren S. Front Syst Neurosci. 2022 Apr 19;16:888461. doi: 10.3389/fnsys.2022.888461. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35520882 Free PMC article. Review. - GluA1 phosphorylation at serine 831 in the lateral amygdala is required for fear renewal.
Lee S, Song B, Kim J, Park K, Hong I, An B, Song S, Lee J, Park S, Kim J, Park D, Lee CJ, Kim K, Shin KS, Tsien RW, Choi S. Lee S, et al. Nat Neurosci. 2013 Oct;16(10):1436-44. doi: 10.1038/nn.3491. Epub 2013 Aug 25. Nat Neurosci. 2013. PMID: 23974710 - Neurobiology of local and intercellular BDNF signaling.
Sasi M, Vignoli B, Canossa M, Blum R. Sasi M, et al. Pflugers Arch. 2017 Jun;469(5-6):593-610. doi: 10.1007/s00424-017-1964-4. Epub 2017 Mar 9. Pflugers Arch. 2017. PMID: 28280960 Free PMC article. Review.
References
- Agranoff BW, Klinger PD. Puromycin Effect on Memory Fixation in Goldfish. Science. 1964;146:952. - PubMed
- Anokhin KV, Tiunova AA, Rose SPR. Reminder effects - reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur J Neurosci. 2002;15:1759–1765. - PubMed
Publication types
MeSH terms
Grants and funding
- R01MH065961/MH/NIMH NIH HHS/United States
- R01 MH065961-07/MH/NIMH NIH HHS/United States
- R01 MH065961-09/MH/NIMH NIH HHS/United States
- R01 MH065961-06A1/MH/NIMH NIH HHS/United States
- R01 MH065961-08/MH/NIMH NIH HHS/United States
- R01 MH065961/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials