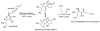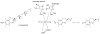Vitamins C and E: beneficial effects from a mechanistic perspective - PubMed (original) (raw)
Review
Vitamins C and E: beneficial effects from a mechanistic perspective
Maret G Traber et al. Free Radic Biol Med. 2011.
Abstract
The mechanistic properties of two dietary antioxidants that are required by humans, vitamins C and E, are discussed relative to their biological effects. Vitamin C (ascorbic acid) is an essential cofactor for α-ketoglutarate-dependent dioxygenases. Examples are prolyl hydroxylases, which play a role in the biosynthesis of collagen and in down-regulation of hypoxia-inducible factor (HIF)-1, a transcription factor that regulates many genes responsible for tumor growth, energy metabolism, and neutrophil function and apoptosis. Vitamin C-dependent inhibition of the HIF pathway may provide alternative or additional approaches for controlling tumor progression, infections, and inflammation. Vitamin E (α-tocopherol) functions as an essential lipid-soluble antioxidant, scavenging hydroperoxyl radicals in a lipid milieu. Human symptoms of vitamin E deficiency suggest that its antioxidant properties play a major role in protecting erythrocyte membranes and nervous tissues. As an antioxidant, vitamin C provides protection against oxidative stress-induced cellular damage by scavenging of reactive oxygen species, by vitamin E-dependent neutralization of lipid hydroperoxyl radicals, and by protecting proteins from alkylation by electrophilic lipid peroxidation products. These bioactivities bear relevance to inflammatory disorders. Vitamin C also plays a role in the function of endothelial nitric oxide synthase (eNOS) by recycling the eNOS cofactor, tetrahydrobiopterin, which is relevant to arterial elasticity and blood pressure regulation. Evidence from plants supports a role for vitamin C in the formation of covalent adducts with electrophilic secondary metabolites. Mechanism-based effects of vitamin C and E supplementation on biomarkers and on clinical outcomes from randomized, placebo-controlled trials are emphasized in this review.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Fig. 1
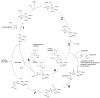
Vitamin C acts as a cofactor for prolyl 4-hydroxylase. The starting point for the catalytic cycle is where the co-substrate, α-ketoglutaric acid, coordinates with the enzyme-bound FeII (step 1). Activation of molecular oxygen (2) and subsequent decarboxylation of α-ketoglutaric acid (3) leads to the formation of the highly energetic FeIV=O reagent that hydroxylates proline residues in procollagen (steps 4 and 5, inner catalytic cycle) [217-219]. If decarboxylation of α-ketoglutaric acid and subsequent formation of the FeIV=O species takes place in the absence of a substrate molecule (proline residue), the FeIV=O species will oxidize a molecule of ascorbic acid in order to regain activity (steps 6 and 7, outer cycle). Thus, ascorbic acid is consumed stoichiometrically in the uncoupled reaction but is not consumed when substrate is available for oxidation. The iron is bound to prolyl 4-hydroxylase through interactions with amino acid residues, His 412, Asp414, and His 483 [10]. The co-substrate, α-ketoglutaric acid, binds to the enzyme-bound iron through two coordination sites as shown. These coordination sites can be occupied by ascorbate upon departure of succinate from the FeIV=O species in the uncoupled reaction (outer cycle).
Fig. 2
Antioxidant effects of vitamins C and E on lipid peroxidation (LPO). The LPO chain reaction can be initiated by many radical species (indicated by R•) and converts LH into LOO•, which attacks another LH generating L• (paths 1 and 2, dotted oval). Ascorbic acid may scavenge the initiating radical species R• and reduce the tocopheroxyl radical, generating the ascorbyl radical, which can be reduced by glutathione dependent enzymes. Key to reaction steps: 1, initiating event; 2, radical propagation reaction; 3, termination of the radical reaction by tocopherol (TocH); 4, dismutation of ascorbyl radicals (Asc•–); 5, reduction of dehydroascorbate (DHAsc) by GSH-dependent dehydroascorbate reductase; 6, GSH peroxidase (GPx); 7, further oxygenation and non-enzymatic cleavage of carbon-carbon bonds yields 4-hydroperoxy-2(E)-nonenal (HPNE); 8, reduction yields 4-hydroxy-2(E)-nonenal (HNE).
Fig. 3
Proposed mechanism of NO biosynthesis by NOS, a heme-containing flavo-enzyme (adapted from [109]). In the first reaction, arginine is hydroxylated to form _N_ω-hydroxyarginine, which is oxidatively converted in the second reaction into NO and citrulline. The oxygen in both reactions originates from a heme FeIV=O species, which is formed by oxygenation of heme-FeII, acceptance of an electron from tetrahydrobiopterin (BH4), protonation and loss of a water molecule (steps 1-4; in step 5, the FeIV-oxo complex arises from the FeV-oxo complex by electron transfer from a ligand nitrogen atom to iron. NO is released in the second reaction via an intermediate FeIII-NO complex. The resulting heme-FeIII species is reduced back to heme-FeII by the flavoprotein domain (step 6). In endothelial NOS, the heme-iron is bound to Cys184 of the enzyme [220].
Fig. 4
Michael adduction of acrolein with ascorbate and subsequent metabolism of the acrolein– ascorbate adduct in cultured human THP-1 monocytes.
Fig. 5
Antioxidant effect of vitamin E. α-Tocopherol reacts with a lipid hydroperoxyl (LOO•) radical. The resultant tocopheryl radical is resonance stabilized, does not react with oxygen (unlike L• radicals) and it can be converted back to α-tocopherol by ascorbate.
Similar articles
- Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids.
Sies H, Stahl W, Sundquist AR. Sies H, et al. Ann N Y Acad Sci. 1992 Sep 30;669:7-20. doi: 10.1111/j.1749-6632.1992.tb17085.x. Ann N Y Acad Sci. 1992. PMID: 1444060 Review. - Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy.
Horton JW. Horton JW. Toxicology. 2003 Jul 15;189(1-2):75-88. doi: 10.1016/s0300-483x(03)00154-9. Toxicology. 2003. PMID: 12821284 Review. - Supplementation of vitamins C and E increases the vitamin E status but does not prevent the formation of oxysterols in the liver of guinea pigs fed an oxidised fat.
Keller U, Brandsch C, Eder K. Keller U, et al. Eur J Nutr. 2004 Dec;43(6):353-9. doi: 10.1007/s00394-004-0481-3. Epub 2004 Feb 17. Eur J Nutr. 2004. PMID: 15309456 - Antioxidant micronutrients for lung disease in cystic fibrosis.
Shamseer L, Adams D, Brown N, Johnson JA, Vohra S. Shamseer L, et al. Cochrane Database Syst Rev. 2010 Dec 8;(12):CD007020. doi: 10.1002/14651858.CD007020.pub2. Cochrane Database Syst Rev. 2010. PMID: 21154377 Updated. Review. - Antioxidant supplementation for lung disease in cystic fibrosis.
Ciofu O, Lykkesfeldt J. Ciofu O, et al. Cochrane Database Syst Rev. 2014 Aug 7;(8):CD007020. doi: 10.1002/14651858.CD007020.pub3. Cochrane Database Syst Rev. 2014. PMID: 25102015 Review.
Cited by
- A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin.
Stephens TJ, Sigler ML, Herndon JH Jr, Dispensa L, Le Moigne A. Stephens TJ, et al. Clin Cosmet Investig Dermatol. 2016 Mar 15;9:63-70. doi: 10.2147/CCID.S98787. eCollection 2016. Clin Cosmet Investig Dermatol. 2016. PMID: 27042135 Free PMC article. - Fat-Soluble Vitamins and the Current Global Pandemic of COVID-19: Evidence-Based Efficacy from Literature Review.
Samad N, Dutta S, Sodunke TE, Fairuz A, Sapkota A, Miftah ZF, Jahan I, Sharma P, Abubakar AR, Rowaiye AB, Oli AN, Charan J, Islam S, Haque M. Samad N, et al. J Inflamm Res. 2021 May 21;14:2091-2110. doi: 10.2147/JIR.S307333. eCollection 2021. J Inflamm Res. 2021. PMID: 34045883 Free PMC article. Review. - Vitamin C intake potentially lowers total cholesterol to improve endothelial function in diabetic patients at increased risk of cardiovascular disease: A systematic review of randomized controlled trials.
Dludla PV, Nkambule BB, Nyambuya TM, Ziqubu K, Mabhida SE, Mxinwa V, Mokgalaboni K, Ndevahoma F, Hanser S, Mazibuko-Mbeje SE, Basson AK, Sabbatinelli J, Tiano L. Dludla PV, et al. Front Nutr. 2022 Oct 31;9:1011002. doi: 10.3389/fnut.2022.1011002. eCollection 2022. Front Nutr. 2022. PMID: 36386907 Free PMC article. - Efficacy and Mechanisms of Antioxidant Compounds and Combinations Thereof against Cisplatin-Induced Hearing Loss in a Rat Model.
Carles L, Gibaja A, Scheper V, Alvarado JC, Almodovar C, Lenarz T, Juiz JM. Carles L, et al. Antioxidants (Basel). 2024 Jun 24;13(7):761. doi: 10.3390/antiox13070761. Antioxidants (Basel). 2024. PMID: 39061830 Free PMC article. - Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies.
Wasti S, Sah N, Mishra B. Wasti S, et al. Animals (Basel). 2020 Jul 24;10(8):1266. doi: 10.3390/ani10081266. Animals (Basel). 2020. PMID: 32722335 Free PMC article. Review.
References
- Gann PH. Randomized trials of antioxidant supplementation for cancer prevention: first bias, now chance--next, cause. Jama. 2009;301:102–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK081761-01A1/DK/NIDDK NIH HHS/United States
- R01 DK067930/DK/NIDDK NIH HHS/United States
- HL081721/HL/NHLBI NIH HHS/United States
- R01 DK067930-04/DK/NIDDK NIH HHS/United States
- DK081761/DK/NIDDK NIH HHS/United States
- DK067930/DK/NIDDK NIH HHS/United States
- R01 HL081721/HL/NHLBI NIH HHS/United States
- R01 DK081761/DK/NIDDK NIH HHS/United States
- R01 HL081721-05/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials