The functional role of reactive stroma in benign prostatic hyperplasia - PubMed (original) (raw)
Review
The functional role of reactive stroma in benign prostatic hyperplasia
Isaiah G Schauer et al. Differentiation. 2011 Nov-Dec.
Abstract
The human prostate gland is one of the only internal organs that continue to enlarge throughout adulthood. The specific mechanisms that regulate this growth, as well as the pathological changes leading to the phenotype observed in the disease benign prostatic hyperplasia (BPH), are essentially unknown. Recent studies and their associated findings have made clear that many complex alterations occur, involving persistent and chronic inflammation, circulating hormonal level deregulation, and aberrant wound repair processes. BPH has been etiologically characterized as a progressive, albeit discontinuous, hyperplasia of both the glandular epithelial and the stromal cell compartments coordinately yielding an expansion of the prostate gland and clinical symptoms. Interestingly, the inflammatory and repair responses observed in BPH are also key components of general wound repair in post-natal tissues. These responses include altered expression of chemokines, cytokines, matrix remodeling factors, chronic inflammatory processes, altered immune surveillance and recognition, as well as the formation of a prototypical 'reactive' stroma, which is similar to that observed across various fibroplasias and malignancies of a variety of tissue sites. Stromal tissue, both embryonic mesenchyme and adult reactive stroma myofibroblasts, has been shown to exert potent and functional regulatory control over epithelial proliferation and differentiation as well as immunoresponsive modulation. Thus, the functional biology of a reactive stroma, within the context of an adult disease typified by epithelial and stromal aberrant hyperplasia, is critical to understand within the context of prostate disease and beyond. The mechanisms that regulate reactive stroma biology in BPH represent targets of opportunity for new therapeutic approaches that may extend to other tissue contexts. Accordingly, this review seeks to address the dissection of important factors, signaling pathways, genes, and other regulatory components that mediate the interplay between epithelium and stromal responses in BPH.
Copyright © 2011 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures
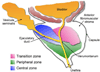
Figure 1. Schematic of the zonal anatomy of the human prostate
As indicated, each zone houses distinct sections of the prostatic urethra. The central zone, where little if any disease develops, houses the ductal tube from the vesicular seminalis. The peripheral zone, the primary site of pre-cancerous and cancerous lesions, houses the descending penile urethra. The transition zone, the only site of benign prostatic hyperplasia, houses the transitional urethra composed of descending bladder and prostatic urethral sections. The verumontanum is the junction between the ejaculatory ducts and the prostatic urethra.
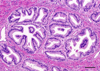
Figure 2. Histomorphology of Nodular Benign Prostatic Hyperplasia
BPH is histologically defined as oblong hyperplastic tissue nodules, most often composed of epithelia and stroma. Pure stromal nodules, lacking any epithelial component, are rare but observed. Nodular growth proximal to the transitional zone urethral tube, constricting urine flow (see Figure 1), accounts for the majority of BPH patient symptoms. H&E, magnification X200, Scale bar = 50µm
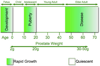
Figure 3. The human prostate continues to enlarge throughout life
The human prostate passes through several phases of rapid growth and relative quiescence, but is the only male organ internal organ that continues to grow through all of adulthood. The various disease states reflect a resumption of rapid growth beyond normal control, including BPH. (Reproduced with kind permission from Springer Science+Business Media: Cell Tissue Research, Early prostate development and its association with late-life prostate disease, Vol 322, 2005, Figure 1 p174, Risbridger G.P. et al., ©Springer-Verlag).
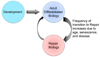
Figure 4. The Biology of Priority
Mammalian embryonic development occurs within the confines of a sterile environment, following a specified pattern of symmetrical and asymmetrical cell division and organ / tissue specification. Adult differentiated biology homeostasis can be disrupted by injury or microbial insult, transitioning to a state of repair-focused biology. Once the insult or injury is sufficiently resolved and tissue is repaired, the biological priority returns back to differentiated functional biology. However, it is likely that senescence, chronic inflammation, and other disease processes associated with aging, affect the efficiency of transition from repair-focused biology back to differentiated homeostasis.
Figure 5. Phenotypic markers of stromal cell differentiation
Myofibroblasts and fibroblasts are both proliferative and synthetic, while smooth muscle is primarily quiescent. In the prostate gland, stromal cell types can be distinguished histologically, and by overlapping expression patterns of vimentin, α-SMA, intracellular synthesis of pro-collagen type 1, deposition of the glycoprotein tenascin-C, and calponin. (With adaptation from The Journal of Urology, Vol 166, Tuxhorn J. et al., Reactive Stroma in Prostate Cancer Progression, Figure 2 on p2474, ©2001 American Urological Association, Inc., with permission from Elsevier).
Similar articles
- Role of stroma in carcinogenesis of the prostate.
Cunha GR, Hayward SW, Wang YZ. Cunha GR, et al. Differentiation. 2002 Dec;70(9-10):473-85. doi: 10.1046/j.1432-0436.2002.700902.x. Differentiation. 2002. PMID: 12492490 Review. - Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia.
Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Schauer IG, et al. Urology. 2008 Jul;72(1):205-13. doi: 10.1016/j.urology.2007.11.083. Epub 2008 Mar 7. Urology. 2008. PMID: 18314176 Free PMC article. - Keratinocyte-derived chemokine induces prostate epithelial hyperplasia and reactive stroma in a novel transgenic mouse model.
Schauer IG, Ressler SJ, Rowley DR. Schauer IG, et al. Prostate. 2009 Mar 1;69(4):373-84. doi: 10.1002/pros.20886. Prostate. 2009. PMID: 19021203 Free PMC article. - Relationship between the prostatic tissue components and natural history of benign prostatic hyperplasia.
Matsuda T, Fujime M, Suda K. Matsuda T, et al. Anal Quant Cytol Histol. 2006 Jun;28(3):121-4. Anal Quant Cytol Histol. 2006. PMID: 16786720 - Benign prostatic hyperplasia: age-related tissue-remodeling.
Untergasser G, Madersbacher S, Berger P. Untergasser G, et al. Exp Gerontol. 2005 Mar;40(3):121-8. doi: 10.1016/j.exger.2004.12.008. Epub 2005 Jan 22. Exp Gerontol. 2005. PMID: 15763388 Review.
Cited by
- Inhibition of chronic prostate inflammation by hyaluronic acid through an immortalized human prostate stromal cell line model.
Liu MC, Chen WH, Chiou CS, Lo WC, Dubey NK, Chen YC, Lai WT, Yeh SD, Chiang HS, Deng WP. Liu MC, et al. PLoS One. 2017 May 30;12(5):e0178152. doi: 10.1371/journal.pone.0178152. eCollection 2017. PLoS One. 2017. PMID: 28558037 Free PMC article. - The Prostate-Associated Gene 4 (PAGE4) Could Play a Role in the Development of Benign Prostatic Hyperplasia under Oxidative Stress.
Li Y, Liu J, Liu D, Wang Z, Zhou Y, Yang S, Guo F, Yang L, Zhang X. Li Y, et al. Oxid Med Cell Longev. 2022 May 19;2022:7041739. doi: 10.1155/2022/7041739. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35633887 Free PMC article. - Phospholipase D inhibitors reduce human prostate cancer cell proliferation and colony formation.
Noble AR, Maitland NJ, Berney DM, Rumsby MG. Noble AR, et al. Br J Cancer. 2018 Jan;118(2):189-199. doi: 10.1038/bjc.2017.391. Epub 2017 Nov 14. Br J Cancer. 2018. PMID: 29136407 Free PMC article. - Activation of innate anti-viral immune response genes in symptomatic benign prostatic hyperplasia.
Madigan AA, Sobek KM, Cummings JL, Green WR, Bacich DJ, O'Keefe DS. Madigan AA, et al. Genes Immun. 2012 Oct;13(7):566-72. doi: 10.1038/gene.2012.40. Epub 2012 Sep 6. Genes Immun. 2012. PMID: 22952051 Free PMC article. - Association between trichomoniasis and prostate and bladder diseases: a population-based case-control study.
Yang HY, Su RY, Chung CH, Huang KY, Lin HA, Wang JY, Chen CC, Chien WC, Lin HC. Yang HY, et al. Sci Rep. 2022 Sep 13;12(1):15358. doi: 10.1038/s41598-022-19561-2. Sci Rep. 2022. PMID: 36100630 Free PMC article.
References
- McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17(3):477–486. - PubMed
- McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15(4):340–345. - PubMed
- Risbridger GP, Almahbobi GA, Taylor RA. Early prostate development and its association with late-life prostate disease. Cell Tissue Res. 2005;322(1):173–181. - PubMed
- Hunter D, Davies J. The Glandular Organ Development Database. Medical Research Council; 1997. An Overview of Prostate Gland Development.
- McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2(1):35–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK083293/DK/NIDDK NIH HHS/United States
- R01 CA058093-18/CA/NCI NIH HHS/United States
- R01 DK083293-02/DK/NIDDK NIH HHS/United States
- R01CA58093/CA/NCI NIH HHS/United States
- R01 DK083293/DK/NIDDK NIH HHS/United States
- R01 CA058093/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical