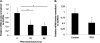Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase - PubMed (original) (raw)
Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase
Alexia Eliades et al. J Biol Chem. 2011.
Abstract
Lysyl oxidase (LOX), a matrix cross-linking protein, is known to be selectively expressed and to enhance a fibrotic phenotype. A recent study of ours showed that LOX oxidizes the PDGF receptor-β (PDGFR-β), leading to amplified downstream signaling. Here, we examined the expression and functions of LOX in megakaryocytes (MKs), the platelet precursors. Cells committed to the MK lineage undergo mitotic proliferation to yield diploid cells, followed by endomitosis and acquisition of polyploidy. Intriguingly, LOX expression is detected in diploid-tetraploid MKs, but scarce in polyploid MKs. PDGFR-BB is an inducer of mitotic proliferation in MKs. LOX inhibition with β-aminopropionitrile reduces PDGFR-BB binding to cells and downstream signaling, as well as its proliferative effect on the MK lineage. Inhibition of LOX activity has no influence on MK polyploidy. We next rationalized that, in a system with an abundance of low ploidy MKs, LOX could be highly expressed and with functional significance. Thus, we resorted to GATA-1(low) mice, where there is an increase in low ploidy MKs, augmented levels of PDGF-BB, and an extensive matrix of fibers. MKs from these mice display high expression of LOX, compared with control mice. Importantly, treatment of GATA-1(low) mice with β-aminopropionitrile significantly improves the bone marrow fibrotic phenotype, and MK number in the spleen. Thus, our in vitro and in vivo data support a novel role for LOX in regulating MK expansion by PDGF-BB and suggest LOX as a new potential therapeutic target for myelofibrosis.
Figures
FIGURE 1.
Differential expression of LOX mRNA levels in megakaryocytes (MKs). A, ex vivo stimulation of bone marrow cells derived from wild-type mice and cultured with 25 ng/ml TPO for 0, 20, and 96 h. MKs were isolated post-treatment via magnetic activated cell sorting columns for qRT-PCR analysis. Data were normalized to GAPDH mRNA. MK purity was evaluated as described under “Experimental Procedures.” B, in vivo effect of tail vein injections of 5 μg/Kg TPO in wild-type mice. Bone marrow was extracted 3 days post-injection, and MKs were isolated for qRT-PCR analysis. Effects of TPO on MK ploidy and platelet counts were confirmed as in a previous study (29). Standard deviation bars represent the mean of three independent experiments; *, p < 0.05.
FIGURE 2.
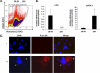
Differential expression of LOX in MKs. A, fluorescence activated cell sorting of MKs based on ploidy on a MoFlo cell sorter. MKs were selected based on CD41-FITC staining and sorted based on Hoechst (DNA) staining in the diploid-tetraploid (2N-4N) and polyploid (≥8N) MK fractions. B, qRT-PCR analysis for LOX and GATA-1 (a marker for MK differentiation) on MK-sorted fractions. Data were normalized to GAPDH mRNA. N.D = not detected. Standard deviation bars represent the mean of four independent experiments; *, p < 0.05. C, staining of low and high ploidy MKs with anti-LOX. Cells were prepared as described under “Experimental Procedures.” DNA was stained with DAPI (blue) to distinguish between typical low ploidy (2N-4N) and high ploidy (≥8N) cells, based on DNA density compared with diploid bone marrow cells, and evaluated as we described previously (58). Immunostaining was performed using rabbit IgG as control and 594 Alexa anti-rabbit secondary antibody to test for antibody specificity (panel I). Panel II: the white arrow depicts a low ploidy MK with pro-LOX staining (red). Within the same field and preparation, a yellow arrow depicts a high ploidy MK with no (or very dim) pro-LOX staining. Data shown represent at least ten slides analyzed, each with ∼105 cytospin cells, from two different experiments.
FIGURE 3.
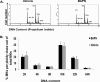
LOX inhibition does not influence MK ploidy. A, BAPN was administered to control mice as described under “Experimental Procedures,” and bone marrow was collected for ploidy analysis. Representative histogram plots for vehicle (H2O) and BAPN administration are shown. B, quantification of ploidy status per group. Statistical analysis was applied using Student's t test. No statistical difference was found (n = 5, p > 0.05). The percentage of CD41-positive cells was estimated as 0.17% (±0.03) for vehicle 0.14% (±0.02) for BAPN-treated mice.
FIGURE 4.
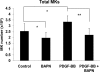
Effect of BAPN on PDGF-BB or control-treated MKs. Bone marrow cultures were treated with 100 μ
m
BAPN and/or 25 ng/ml PDGF-BB for 3 days in serum-free media. FACS analysis was performed, and total MKs number and percentage were calculated based on total bone marrow cell count. Standard deviation bars represent the mean of ten independent experiments, n = 10; *, p < 0.05, **, p < 0.001.
FIGURE 5.
Effect of LOX inhibition on PDGFR-β-mediated signaling. Western blot analysis of PDGF-Rβ downstream targets Akt and ERK1/2 in MKs. Bone marrow cells were stimulated with 25 ng/ml TPO for 3 days in the presence or absence of 100 μ
m
BAPN, followed by magnetic activated cell sorting purification of MKs that were further cultured in serum-free media in the presence or absence of 100 μ
m
BAPN for 16 h. MKs were next stimulated with 50 ng/ml PDGF-BB and collected for analysis at different time points. Blots were probed with the indicated antibodies, with t indicating total protein and p indicating the phosphorylated form. Probing with anti-β-actin was used as loading control. Shown here is a representative Western blot out of three independent experiments.
FIGURE 6.
LOX levels in MKs of GATA-1low mice. A, ploidy analysis of wild-type and GATA-1low MKs. Cells were stained with CD41-FITC antibody and propidium iodide (DNA). Flow cytometry was performed on a BD Biosciences Calibur using CellQuest. Representative histograms are shown per group (wild-type, GATA-1low male littermates). B, quantification of MK percentage (CD41-positive cells) of diploid-tetraploid and polyploid fractions. C, platelet numbers of wild-type and GATA-1low MKs. Blood was collected via heart puncture and platelet number was assessed on a Hemavet blood analyzer. D, qRT-PCR on isolated MKs to evaluate LOX and GATA-1 gene expression. Data were normalized to GAPDH mRNA. Standard deviation bars represent the mean of five independent experiments; *, p < 0.05; **, p < 0.001.
FIGURE 7.
Effect of LOX inhibition on splenic GATA-1low MKs. Spleens were harvested from wild-type and GATA-1low mice (male littermates) that were in either control or BAPN-drinking water treatment as described under “Experimental Procedures.” A, measurement of spleen mass. Standard deviation bars represent the mean of n = 6 mice per group (wild-type/BAPN groups, n = 4 mice); **, p < 0.001. B, representative spleen sections stained with hematoxylin and eosin. MKs were readily detected based on the large nucleus and morphology, and our method of detection was further confirmed by marking MKs with anti-CD41 as shown in
supplemental Fig. S7
. Original magnification, 200×. C, quantification of spleen MKs in a total area of 0.21 mm2. Results are presented as the mean of three mice per group, with nine slides analyzed per mouse; *, p < 0.05.
FIGURE 8.
Effect of LOX inhibition on marrow fibrosis in vivo. A, representative hematoxylin & eosin (H&E, left column) and Gomori silver (right column) staining of longitudinal sections of femurs from wild-type and GATA-1low (male littermates), control, or BAPN-treated mice (10.5 weeks old at the time of collection), as described under “Experimental Procedures.” Original magnification for the left column was 400× and for the right column are 600×. Arrows indicate the large presence of MKs (H&E stain) and the accumulation of reticulin fibers (Gomori) in the GATA-1low mice. As expected, no such fibers were detected in wild-type mice. Counting morphologically recognizable MKs in eight sections per mouse, using histological approaches described earlier (–39), showed no significant differences in MK number in bone marrow sections of control and BAPN-treated mice. Similar results were obtained in a total of five mice per group. MK counting was further confirmed by flow cytometry analysis as in Fig. 3 (data not shown). B, quantification of fibrosis in BAPN- or vehicle-treated GATA-1low mice. Fibers were measured in arbitrary units (as described under “Experimental Procedures”) from stained sections. Data are represented as absolute values (top panel) or as mean percent change compared with values recorded from vehicle-treated GATA-1low mice (bottom panel). The mean values were obtained from five mice per group. Scale bars (10 μm) are at the upper left corner of each column. Results are presented as absolute values and percentage change; n = 5; *, p < 0.05.
Similar articles
- Megakaryocyte polyploidy is inhibited by lysyl oxidase propeptide.
Eliades A, Papadantonakis N, Matsuura S, Mi R, Bais MV, Trackman P, Ravid K. Eliades A, et al. Cell Cycle. 2013 Apr 15;12(8):1242-50. doi: 10.4161/cc.24312. Epub 2013 Mar 21. Cell Cycle. 2013. PMID: 23518500 Free PMC article. - Lysyl oxidase oxidizes cell membrane proteins and enhances the chemotactic response of vascular smooth muscle cells.
Lucero HA, Ravid K, Grimsby JL, Rich CB, DiCamillo SJ, Mäki JM, Myllyharju J, Kagan HM. Lucero HA, et al. J Biol Chem. 2008 Aug 29;283(35):24103-17. doi: 10.1074/jbc.M709897200. Epub 2008 Jun 27. J Biol Chem. 2008. PMID: 18586678 Free PMC article. - Kinetics of endomitosis in primary murine megakaryocytes.
Carow CE, Fox NE, Kaushansky K. Carow CE, et al. J Cell Physiol. 2001 Sep;188(3):291-303. doi: 10.1002/jcp.1120. J Cell Physiol. 2001. PMID: 11473355 - Megakaryocytes and platelets in myeloproliferative disorders.
Briere J, Kiladjian JJ, Peynaud-Debayle E. Briere J, et al. Baillieres Clin Haematol. 1997 Feb;10(1):65-88. doi: 10.1016/s0950-3536(97)80051-0. Baillieres Clin Haematol. 1997. PMID: 9154316 Review. - Protein arginine methyltransferase 1 in the generation of immune megakaryocytes: A perspective review.
Zhao X, Chong Z, Chen Y, Zheng XL, Wang QF, Li Y. Zhao X, et al. J Biol Chem. 2022 Nov;298(11):102517. doi: 10.1016/j.jbc.2022.102517. Epub 2022 Sep 21. J Biol Chem. 2022. PMID: 36152748 Free PMC article. Review.
Cited by
- Dynamins 2 and 3 control the migration of human megakaryocytes by regulating CXCR4 surface expression and ITGB1 activity.
Suraneni PK, Corey SJ, Hession MJ, Ishaq R, Awomolo A, Hasan S, Shah C, Liu H, Wickrema A, Debili N, Crispino JD, Eklund EA, Chen Y. Suraneni PK, et al. Blood Adv. 2018 Dec 11;2(23):3540-3552. doi: 10.1182/bloodadvances.2018021923. Blood Adv. 2018. PMID: 30538113 Free PMC article. - GATA1 insufficiencies in primary myelofibrosis and other hematopoietic disorders: consequences for therapy.
Ling T, Crispino JD, Zingariello M, Martelli F, Migliaccio AR. Ling T, et al. Expert Rev Hematol. 2018 Mar;11(3):169-184. doi: 10.1080/17474086.2018.1436965. Epub 2018 Feb 19. Expert Rev Hematol. 2018. PMID: 29400094 Free PMC article. Review. - Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies.
Zahr AA, Salama ME, Carreau N, Tremblay D, Verstovsek S, Mesa R, Hoffman R, Mascarenhas J. Zahr AA, et al. Haematologica. 2016 Jun;101(6):660-71. doi: 10.3324/haematol.2015.141283. Haematologica. 2016. PMID: 27252511 Free PMC article. Review. - Megakaryocyte Contribution to Bone Marrow Fibrosis: many Arrows in the Quiver.
Malara A, Abbonante V, Zingariello M, Migliaccio A, Balduini A. Malara A, et al. Mediterr J Hematol Infect Dis. 2018 Nov 1;10(1):e2018068. doi: 10.4084/MJHID.2018.068. eCollection 2018. Mediterr J Hematol Infect Dis. 2018. PMID: 30416700 Free PMC article. Review. - Cancer-associated fibroblasts in hematologic malignancies: elucidating roles and spotlighting therapeutic targets.
Ding Z, Shi R, Hu W, Tian L, Sun R, Wu Y, Zhang X. Ding Z, et al. Front Oncol. 2023 Sep 6;13:1193978. doi: 10.3389/fonc.2023.1193978. eCollection 2023. Front Oncol. 2023. PMID: 37746306 Free PMC article. Review.
References
- Kagan H. M., Li W. (2003) J. Cell Biochem. 88, 660–672 - PubMed
- Trackman P. C., Bedell-Hogan D., Tang J., Kagan H. M. (1992) J. Biol. Chem. 267, 8666–8671 - PubMed
- Rodríguez C., Martínez-González J., Raposo B., Alcudia J. F., Guadall A., Badimon L. (2008) Cardiovasc. Res. 79, 7–13 - PubMed
- Gilad G. M., Kagan H. M., Gilad V. H. (2005) Neurosci. Lett 376, 210–214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE014066/DE/NIDCR NIH HHS/United States
- R01 DE014066-09/DE/NIDCR NIH HHS/United States
- R01 HL080442/HL/NHLBI NIH HHS/United States
- HL80442/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous