Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis - PubMed (original) (raw)
Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis
Geber Peña et al. J Immunol. 2011.
Abstract
Many anti-inflammatory strategies that are successful in treating sepsis in healthy animals fail in clinical trials, in part because sepsis normally involves immunocompromised patients, and massive lymphocyte apoptosis prevents immunomodulation. In this article, we report a new set of regulatory lymphocytes that are able to re-establish the cholinergic anti-inflammatory modulation and to provide therapeutic advantages in sepsis. The vagus nerve controls inflammation in healthy, but not in septic, mice. Likewise, vagus nerve and cholinergic agonists fail to control inflammation in splenectomized and nude animals. Unlike typical suppressor CD25(+) cells, CD4(+)CD25(-) lymphocytes re-establish the anti-inflammatory potential of the vagus nerve and cholinergic agonists in immunocompromised and septic animals. These cholinergic lymphocytes re-establish splenic protection and the potential of cholinergic agonists to rescue immunocompromised animals from established sepsis. The study results revealed these new regulatory lymphocytes as, to our knowledge, the first known physiological target for neuromodulation of the innate immune responses and a potential therapeutic target for sepsis.
Figures
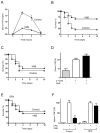
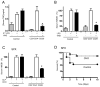
Figure 1. Vagus nerve stimulation prevents lethal endotoxemia
Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 20 mins before LPS (6mg/Kg, ip). (A) Serum TNF was analyzed by ELISA (n=4; One-way ANOVA with Bonferroni’s corrections). (B) Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group). (C) Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 6 hrs after the LPS challenge. Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group). (D) Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 6 hrs after the original LPS challenge. Then, animals received a second dose of LPS (2ndLPS), and serum TNF concentrations were measured at 90 mins later (n=4; One-way ANOVA with Bonferroni’s corrections). (E) Splenectomized (SPX) animals underwent sham (Control) or vagus nerve stimulation (VNS) started 20 mins before LPS (6mg/Kg, ip). Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group). (F) Control (laparotomy) or splenectomized (SPX) animals underwent sham surgery or vagus nerve stimulation (VNS) and LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
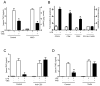
Figure 2. Vagus nerve immunomodulation requires splenic T lymphocytes
(A) Wild-type (Control) or Mast cell-knockout (MKO) mice received sham or vagus nerve stimulation (VNS) and LPS(6mg/Kg, ip). (B) BALB/c mice received vehicle or LPS (6mg/Kg, ip), and apoptosis in different cell types was analyzed by double-staining in FACS. (C) BALB/c mice were treated with vehicle (control) or the neutralizing anti-CD3 antibody (Anti-CD3, 20 mg/kg) 24 hrs before endotoxemia. (D) Control or nude mice received LPS, and sham surgery or vagus nerve stimulation (VNS). In all the experiments, serum TNF concentrations were analyzed at 90 mins and represented as percent of the LPS response. # represents p<0.05 vs. LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
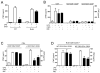
Figure 3. CD3+CD4+CD25− lymphocytes reestablish the anti-inflammatory potential of α7nR-agonists in splenocytes
(A) Primary cultures of splenocytes from wild-type (WT) or nude mice were treated with α7nR-agonist choline (CHO) and/or LPS. (B) Splenocytes from wild-type mice were fractionated to isolate CD4−, CD3+CD4+CD25+ or CD3+CD4+CD25− cells, stimulated with endotoxin, and treated with choline (CHO, 20mM) or nicotine (NIC,2uM). (C) Purified CD4− splenocytes were co-cultured with CD3+CD4+CD25+ or CD3+CD4+CD25− splenocytes and treated with choline (CHO, 20mM) or nicotine (NIC,2uM). (D) Splenocytes from nude mice were co-cultured with CD3+CD4+CD25+ or CD3+CD4+CD25− splenocytes from wild-type animals, stimulated with endotoxin, and treated with the indicated agonists. In all the experiments, splenocytes were pretreated with α7nR-agonists 30 mins prior LPS (100ng/mL). TNF concentrations were analyzed by ELISA in the conditioned media at 3 hours after stimulation. Graph represents the data of three different experiments in mean ± STD. * represents p<0.01 vs. LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
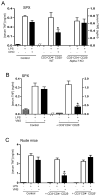
Figure 4. CD3+CD4+CD25− lymphocytes reestablish vagal anti-inflammatory mechanism in Nude mice
(A) Splenectomized (SPX) animals received CD3+CD4+CD25− lymphocytes from wild-type (WT) or α7nR-knockout (Alpha7KO) animals. 24 hrs later, animals were treated with α7nR-agonist, choline (CHO, 400ug/kg; ip) and endotoxemia (LPS). (B) Splenectomized (SPX) animals received vehicle (control) or CD4+CD25− splenocytes from control animals, 24 hrs before vagus nerve stimulation (VNS) and endotoxemia (LPS). (C) Nude mice received vehicle (control), CD3+CD4+CD25+ or CD3+CD4+CD25− lymphocytes from control wild-type animals, 24 hrs before vagus nerve stimulation (VNS) and endotoxemia (LPS). In all the experiments, serum TNF levels were analyzed by ELISA at 90 mins after the endotoxic challenge. Graphs represent the results of four different animals in mean ± STD. * represents p<0.01 vs. LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
Figure 5
CD3+CD4+CD25− lymphocytes reestablish cholinergic protection against sepsis and rescue animals from established sepsis. (A) Mice received vehicle (control) or CD3+CD4+CD25− lymphocytes 6 hrs after endotoxemia (LPS; 6mg/Kg; ip). One hour later, animals underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 20 mins before a second dose of LPS (2ndLPS) (n=4; One-way ANOVA with Bonferroni’s corrections). (B) Mice received vehicle (control) or CD4+CD25− lymphocytes, one day after endotoxemia (LPS; 6mg/Kg; ip). Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS), and serum HMGB1 levels were analyzed at 44 hrs (n=4; One-way ANOVA with Bonferroni’s corrections). (C, D) Splenectomized (SPX) underwent polymicrobial sepsis induced by cecal ligation and puncture (CLP). One day later, mice received vehicle (control) or CD3+CD4+CD25− lymphocytes. Mice were treated with vehicle (control) or choline (CHO, 400ug/kg;ip), and serum HMGB1 levels were analyzed at 44 hrs (n=4; One-way ANOVA with Bonferroni’s corrections). (D) Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group).
Similar articles
- β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system.
Vida G, Peña G, Kanashiro A, Thompson-Bonilla Mdel R, Palange D, Deitch EA, Ulloa L. Vida G, et al. FASEB J. 2011 Dec;25(12):4476-85. doi: 10.1096/fj.11-191007. Epub 2011 Aug 12. FASEB J. 2011. PMID: 21840939 Free PMC article. - Constitutive Vagus Nerve Activation Modulates Immune Suppression in Sepsis Survivors.
Rana M, Fei-Bloom Y, Son M, La Bella A, Ochani M, Levine YA, Chiu PY, Wang P, Chavan SS, Volpe BT, Sherry B, Diamond B. Rana M, et al. Front Immunol. 2018 Sep 6;9:2032. doi: 10.3389/fimmu.2018.02032. eCollection 2018. Front Immunol. 2018. PMID: 30237803 Free PMC article. - Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis.
Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Huston JM, et al. J Exp Med. 2006 Jul 10;203(7):1623-8. doi: 10.1084/jem.20052362. Epub 2006 Jun 19. J Exp Med. 2006. PMID: 16785311 Free PMC article. - The role of the cholinergic anti-inflammatory pathway in septic cardiomyopathy.
Wang W, Xu H, Lin H, Molnar M, Ren H. Wang W, et al. Int Immunopharmacol. 2021 Jan;90:107160. doi: 10.1016/j.intimp.2020.107160. Epub 2020 Nov 24. Int Immunopharmacol. 2021. PMID: 33243604 Review. - The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis.
Huston JM. Huston JM. Surg Infect (Larchmt). 2012 Aug;13(4):187-93. doi: 10.1089/sur.2012.126. Epub 2012 Aug 22. Surg Infect (Larchmt). 2012. PMID: 22913335 Review.
Cited by
- Nerve Stimulation: Immunomodulation and Control of Inflammation.
Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Ulloa L, et al. Trends Mol Med. 2017 Dec;23(12):1103-1120. doi: 10.1016/j.molmed.2017.10.006. Epub 2017 Nov 20. Trends Mol Med. 2017. PMID: 29162418 Free PMC article. Review. - Modulation of experimental arthritis by vagal sensory and central brain stimulation.
Bassi GS, Dias DPM, Franchin M, Talbot J, Reis DG, Menezes GB, Castania JA, Garcia-Cairasco N, Resstel LBM, Salgado HC, Cunha FQ, Cunha TM, Ulloa L, Kanashiro A. Bassi GS, et al. Brain Behav Immun. 2017 Aug;64:330-343. doi: 10.1016/j.bbi.2017.04.003. Epub 2017 Apr 6. Brain Behav Immun. 2017. PMID: 28392428 Free PMC article. - Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis.
Gatta V, Mengod G, Reale M, Tata AM. Gatta V, et al. Biomedicines. 2020 Jun 8;8(6):153. doi: 10.3390/biomedicines8060153. Biomedicines. 2020. PMID: 32521719 Free PMC article. Review. - Effects of anti-inflammatory vagus nerve stimulation in endotoxemic rats on blood and spleen lymphocyte subsets.
Mihaylova S, Schweighöfer H, Hackstein H, Rosengarten B. Mihaylova S, et al. Inflamm Res. 2014 Aug;63(8):683-90. doi: 10.1007/s00011-014-0741-5. Epub 2014 May 7. Inflamm Res. 2014. PMID: 24802890 - Exercise activates vagal induction of dopamine and attenuates systemic inflammation.
Shimojo G, Joseph B, Shah R, Consolim-Colombo FM, De Angelis K, Ulloa L. Shimojo G, et al. Brain Behav Immun. 2019 Jan;75:181-191. doi: 10.1016/j.bbi.2018.10.005. Epub 2018 Oct 27. Brain Behav Immun. 2019. PMID: 30394312 Free PMC article.
References
- Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. Jama. 1997;278:234–240. - PubMed
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
- Angus DC. Caring for the critically ill patient: challenges and opportunities. Jama. 2007;298:456–458. - PubMed
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. - PubMed
- Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4:378–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials