The defective nuclear lamina in Hutchinson-gilford progeria syndrome disrupts the nucleocytoplasmic Ran gradient and inhibits nuclear localization of Ubc9 - PubMed (original) (raw)
The defective nuclear lamina in Hutchinson-gilford progeria syndrome disrupts the nucleocytoplasmic Ran gradient and inhibits nuclear localization of Ubc9
Joshua B Kelley et al. Mol Cell Biol. 2011 Aug.
Abstract
The mutant form of lamin A responsible for the premature aging disease Hutchinson-Gilford progeria syndrome (termed progerin) acts as a dominant negative protein that changes the structure of the nuclear lamina. How the perturbation of the nuclear lamina in progeria is transduced into cellular changes is undefined. Using patient fibroblasts and a variety of cell-based assays, we determined that progerin expression in Hutchinson-Gilford progeria syndrome inhibits the nucleocytoplasmic transport of several factors with key roles in nuclear function. We found that progerin reduces the nuclear/cytoplasmic concentration of the Ran GTPase and inhibits the nuclear localization of Ubc9, the sole E2 for SUMOylation, and of TPR, the nucleoporin that forms the basket on the nuclear side of the nuclear pore complex. Forcing the nuclear localization of Ubc9 in progerin-expressing cells rescues the Ran gradient and TPR import, indicating that these pathways are linked. Reducing nuclear SUMOylation decreases the nuclear mobility of the Ran nucleotide exchange factor RCC1 in vivo, and the addition of SUMO E1 and E2 promotes the dissociation of RCC1 and Ran from chromatin in vitro. Our data suggest that the cellular effects of progerin are transduced, at least in part, through reduced function of the Ran GTPase and SUMOylation pathways.
Figures
Fig. 1.
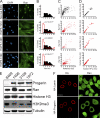
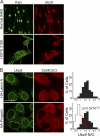
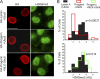
The Ran protein gradient in interphase cells depends on the nuclear lamina and is correlated with markers of heterochromatin. (A) Ran distribution in primary fibroblasts from a control individual (Normal 8469) and three progeria patients (HGPS 1972, HGPS 1498, and HGPS 3199). DAPI, 4′,6-diamidino-2-phenylindole. (B) Histograms (bin size = 0.5) of Ran N/C ratios from control (Normal 8469) (black bars; n = 293) and HGPS patient (HGPS 1972, HGPS 1498, and HGPS 3199) (red lines; n = 264, 53, and 207, respectively) cells. (C) Ran N/C values plotted as a function of nuclear H3K9me3 levels (Spearman's rank correlation coefficient [ρ] values of 0.81 for Normal 8469, 0.74 for HGPS 1972, 0.81 for HGPS 1498, and 0.29 for HGPS 3199). (D) Nuclear Ran values plotted as a function of nuclear HP1γ levels (ρ values of 0.81 for Normal 8469, 0.74 for HGPS 1972, 0.81 for HGPS 1498, and 0.29 for HGPS 3199; n = 64, 50, 40, and 108, respectively). AU, arbitrary units. (E) Immunoblotting of primary fibroblasts. (F) Colocalization of transiently transfected HA-tagged WT lamin A and HA-progerin (red) with endogenous Ran (green) in HeLa cells. Scale bars, 20 μm.
Fig. 2.
The importin β-dependent import pathway is functional in HGPS fibroblasts. (A) Rate of importin β-dependent transport measured in living cells. A fluorescent reporter protein (Alexa 544-labeled IBB-β-galactosidase) that undergoes importin β-dependent import was injected into the cytoplasm of control (Normal 8469) and progeria (HGPS 1498) fibroblasts. For each injected cell, the initial rate was plotted versus the initial concentration. The slopes of the solid lines represent the relationship between the import rate and the reporter protein concentration. Dashed lines represent 95% confidence intervals. (B) Images of Normal 8469 and HGPS 1498 fibroblasts 3 s and 117 s after microinjection. Scale bar, 20 μm.
Fig. 3.
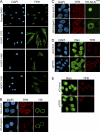
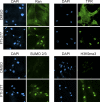
Progerin inhibits TPR import. (A) Distribution of endogenous TPR in control (Normal 8469) and progeria (HGPS 1972, HGPS 1498, and HGPS 3199) fibroblasts. (B) HA-lamin A and HA-progerin transfection in HeLa cells costained for endogenous TPR (red) and HA (green). (C) Cotransfection of progerin and a reporter protein that contains the NLS from TPR (pyruvate kinase) (PK-NLSTPR) followed by localization of endogenous TPR (red) and the PK reporter (green). (D) siRNA-mediated knockdown of NTF2 in HeLa cells and localization of endogenous Ran (green) and TPR (red). (E) siRNA-mediated knockdown of TPR in HeLa cells with localization of endogenous Ran (green) and TPR (red). Scale bars, 20 μm.
Fig. 4.
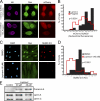
SUMO2/3 levels in the nucleus are reduced in response to progerin expression. (A) Overlay of SUMO1 and SUMO2/3 distributions (red) with HA-progerin and HA-WT lamin A (green) in HeLa cells. (B) SUMO2/3 (red) and Ran (green) levels in control (Normal 8469) and progeria (HGPS 1972, HGPS 1498, and HGPS 3199) fibroblasts. (C) Histograms (bin size = 1) of nuclear SUMO2/3 levels from healthy (Normal 8469) (black bars; n = 54) and HGPS patient (HGPS 1972, HGPS 1498, and HGPS 3199) (red lines; n = 56, 65, and 101, respectively) cells. (D) Ran N/C ratio values plotted as a function of nuclear SUMO2/3 levels (ρ values of 0.19 for Normal 8469, 0.82 for HGPS 1972, 0.70 for HGPS 1498, and 0.53 for HGPS 3199). Scale bars, 20 μm.
Fig. 5.
Membrane attachment underlies progerin effects on SUMOylation. (A) Images from HeLa cells triple labeled for HA-progerin and HA-WT lamin A (purple), endogenous Ran (green), and mCherry-SUMO2 (red). (B) Histograms (bin size = 0.1) of Cherry-SUMO2 levels (nuclear/total) in progerin- and lamin A-transfected cells. (C) Endogenous Ran and SUMO2/3 detected in primary human fibroblasts (Normal 8469) treated with lopinavir. (D) Histograms (bin size = 1) of SUMO2/3 levels in cells treated with lopinavir or DMSO. (E) Immunoblotting of primary human fibroblasts treated with lopinavir for 4 days. Total cell extracts were probed with a pan-lamin antibody that recognizes lamin A/C and an antibody that selectively recognizes unprocessed, prelamin A. Scale bars, 20 μm (A and C).
Fig. 6.
Inhibition of SUMOylation disrupts the Ran protein gradient. (A) HeLa cells were transfected with FLAG-tagged forms of WT Ubc9, a catalytic mutant of Ubc9 (C93S) that acts as a DN protein, and the SENP CD. Double-label IF microscopy for FLAG (red) and endogenous Ran (green) showed that the Ran protein gradient is disrupted by the expression of Ubc9 C93S and the SENP CD (left) under conditions where these factors reduce nuclear SUMO2/3 levels (right). (B and C) The SENP CD (B) and HA-progerin (C) do not disrupt endogenous RanGAP targeting to the NPC. Scale bars, 20 μm.
Fig. 7.
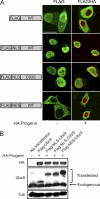
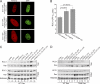
Reduced nuclear localization of Ubc9 in HGPS. (A) Endogenous Ran (green) and Ubc9 (red) in control (Normal 8469) and progeria (HGPS 3199) fibroblasts. (B) Localization of endogenous Ubc9 (green) and SUMO2/3 (red) in HeLa cells cotransfected with HA-WT lamin A and HA-progerin. Histograms (bin size = 0.25) of Ubc9 N/C ratios in HeLa cells expressing HA-lamin A (black bars; n = 43) and HA-progerin (red lines; n = 62). Scale bars, 20 μm.
Fig. 8.
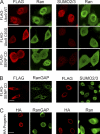
Transport signal fusions that direct nuclear and cytoplasmic targeting of Ubc9. (A) FLAG-tagged SV40 NLS and PKI NES fusions with Ubc9 (green) were expressed in HeLa cells in the absence and presence of HA-progerin (red). Scale bar, 20 μm. (B) Immunoblotting showing the expression levels of the Ubc9 transport signal fusions relative to those of endogenous Ubc9.
Fig. 9.
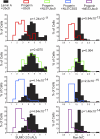
Forcing nuclear localization of Ubc9 rescues the Ran gradient in progerin-expressing cells. HeLa cells were cotransfected with HA-WT lamin A and HA-progerin, together with the transport signal fusions of Ubc9. Histograms show the nuclear levels of SUMO2 (bin size = 0.5) and Ran N/C levels (bin size = 0.25) in HA-positive cells expressing ectopic forms of Ubc9.
Fig. 10.
Nuclear localization of Ubc9 restores TPR import in cells expressing progerin. (A) IF localization of HA-progerin and endogenous TPR in cells cotransfected with Ubc9. Scale bar, 20 μm. (B) Histograms showing TPR N/C levels (bin size = 0.5) in HA-positive cells expressing engineered forms of Ubc9.
Fig. 11.
Nuclear localization of Ubc9 restores H3K9me3 levels in cells expressing progerin. (A) IF localization of HA-progerin and nuclear H3K9me3 in cells cotransfected with Ubc9. Scale bar, 20 μm. (B) Histograms showing H3K9me3 levels (bin size = 1) in HA-positive cells expressing engineered forms of Ubc9.
Fig. 12.
Farnesylation is required for the inhibitory effects of progerin on Ran, TPR, SUMO2/3, and H3K9me3. Progeria fibroblasts (HGPS 3199) were treated with FTI-277 (3 μM) or DMSO (0.1%) for 72 h and examined by IF microscopy for the indicated proteins. Scale bar, 20 μm.
Fig. 13.
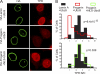
SUMOylation regulates RCC1-chromatin interactions. (A) Localizations of mCherry-SUMO2 (red) and RCC1-GFP (green) in HeLa cells cotransfected HA-WT lamin A, HA-progerin, or the FLAG-SENP CD. (B) Nuclear mobility of RCC1-GFP measured by FRAP of cells expressing WT lamin A (n = 31), progerin (n = 24), or the SENP CD (n = 29). Error bars represent standard errors of the means (SEM). (C) SUMOylation-induced dissociation of RCC1 from chromatin. HeLa cell chromatin prebound with His-Ran was incubated with the combinations of recombinant factors indicated. Following a centrifugation step, RCC1 and Ran in supernatant and pellet fractions were analyzed by immunoblotting. Histones in the pellet fraction were visualized by Ponceau S staining. (D) The T24N form of Ran inhibits the SUMOylation-induced dissociation of RCC1 from chromatin. HeLa cell chromatin prebound with GST-Ran (WT and T24N) was treated as described above (C).
Fig. 14.
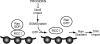
Working model for how progerin disrupts the Ran gradient in HGPS. The expression of the progerin form of lamin A in HGPS inhibits Ubc9 import, which results in reduced levels of nuclear SUMOylation by SUMO2/3, reduced RCC1 function, and a disruption of the Ran protein gradient. Although classical importin β-dependent import is largely unaffected by progerin, the nuclear import of TPR is defective because of its sensitivity to the Ran protein gradient. TPR modulates multiple nuclear pathways, including those important for posttranscriptional gene regulation (25, 26, 31, 47, 66, 78), raising the possibility that the absence of TPR from the NPC might contribute to some of the gene expression changes associated with HGPS.
Similar articles
- A pathway linking oxidative stress and the Ran GTPase system in progeria.
Datta S, Snow CJ, Paschal BM. Datta S, et al. Mol Biol Cell. 2014 Apr;25(8):1202-15. doi: 10.1091/mbc.E13-07-0430. Epub 2014 Feb 12. Mol Biol Cell. 2014. PMID: 24523287 Free PMC article. - Defective nuclear import of Tpr in Progeria reflects the Ran sensitivity of large cargo transport.
Snow CJ, Dar A, Dutta A, Kehlenbach RH, Paschal BM. Snow CJ, et al. J Cell Biol. 2013 May 13;201(4):541-57. doi: 10.1083/jcb.201212117. Epub 2013 May 6. J Cell Biol. 2013. PMID: 23649804 Free PMC article. - A nuclear lamina-chromatin-Ran GTPase axis modulates nuclear import and DNA damage signaling.
Dworak N, Makosa D, Chatterjee M, Jividen K, Yang CS, Snow C, Simke WC, Johnson IG, Kelley JB, Paschal BM. Dworak N, et al. Aging Cell. 2019 Feb;18(1):e12851. doi: 10.1111/acel.12851. Epub 2018 Dec 19. Aging Cell. 2019. PMID: 30565836 Free PMC article. - Towards delineating the chain of events that cause premature senescence in the accelerated aging syndrome Hutchinson-Gilford progeria (HGPS).
Dreesen O. Dreesen O. Biochem Soc Trans. 2020 Jun 30;48(3):981-991. doi: 10.1042/BST20190882. Biochem Soc Trans. 2020. PMID: 32539085 Free PMC article. Review. - Hutchinson-Gilford progeria syndrome through the lens of transcription.
Prokocimer M, Barkan R, Gruenbaum Y. Prokocimer M, et al. Aging Cell. 2013 Aug;12(4):533-43. doi: 10.1111/acel.12070. Epub 2013 Apr 19. Aging Cell. 2013. PMID: 23496208 Review.
Cited by
- Interdependence between Nuclear Pore Gatekeepers and Genome Caretakers: Cues from Genome Instability Syndromes.
Larizza L, Colombo EA. Larizza L, et al. Int J Mol Sci. 2024 Aug 29;25(17):9387. doi: 10.3390/ijms25179387. Int J Mol Sci. 2024. PMID: 39273335 Free PMC article. Review. - Arterial stiffness and cardiac dysfunction in Hutchinson-Gilford Progeria Syndrome corrected by inhibition of lysyl oxidase.
von Kleeck R, Roberts E, Castagnino P, Bruun K, Brankovic SA, Hawthorne EA, Xu T, Tobias JW, Assoian RK. von Kleeck R, et al. Life Sci Alliance. 2021 Mar 9;4(5):e202000997. doi: 10.26508/lsa.202000997. Print 2021 May. Life Sci Alliance. 2021. PMID: 33687998 Free PMC article. - Fluorescence-based quantification of nucleocytoplasmic transport.
Kelley JB, Paschal BM. Kelley JB, et al. Methods. 2019 Mar 15;157:106-114. doi: 10.1016/j.ymeth.2018.11.002. Epub 2018 Nov 10. Methods. 2019. PMID: 30419335 Free PMC article. - Location, location, location: subcellular protein partitioning in proteostasis and aging.
Kumar AV, Lapierre LR. Kumar AV, et al. Biophys Rev. 2021 Nov 19;13(6):931-941. doi: 10.1007/s12551-021-00890-x. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35047088 Free PMC article. Review. - Genotype-phenotype analysis of LMNA-related diseases predicts phenotype-selective alterations in lamin phosphorylation.
Lin EW, Brady GF, Kwan R, Nesvizhskii AI, Omary MB. Lin EW, et al. FASEB J. 2020 Jul;34(7):9051-9073. doi: 10.1096/fj.202000500R. Epub 2020 May 15. FASEB J. 2020. PMID: 32413188 Free PMC article.
References
- Aebi U., Cohn J., Buhle L., Gerace L. 1986. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323:560–564 - PubMed
- Akhtar A., Gasser S. M. 2007. The nuclear envelope and transcriptional control. Nat. Rev. Genet. 8:507–517 - PubMed
- Alcendor R., et al. 2007. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 100:1512–1521 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous