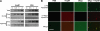Sequestration of toxic oligomers by HspB1 as a cytoprotective mechanism - PubMed (original) (raw)
Sequestration of toxic oligomers by HspB1 as a cytoprotective mechanism
Juhi Ojha et al. Mol Cell Biol. 2011 Aug.
Abstract
Small heat shock proteins (sHsps) are molecular chaperones that protect cells from cytotoxic effects of protein misfolding and aggregation. HspB1, an sHsp commonly associated with senile plaques in Alzheimer's disease (AD), prevents the toxic effects of Aβ aggregates in vitro. However, the mechanism of this chaperone activity is poorly understood. Here, we observed that in two distinct transgenic mouse models of AD, mouse HspB1 (Hsp25) localized to the penumbral areas of plaques. We have demonstrated that substoichiometric amounts of human HspB1 (Hsp27) abolish the toxicity of Aβ oligomers on N2a (mouse neuroblastoma) cells. Using biochemical methods, spectroscopy, light scattering, and microscopy methods, we found that HspB1 sequesters toxic Aβ oligomers and converts them into large nontoxic aggregates. HspB1 was overexpressed in N2a cells in response to treatment with Aβ oligomers. Cultured neurons from HspB1-deficient mice were more sensitive to oligomer-mediated toxicity than were those from wild-type mice. Our results suggest that sequestration of oligomers by HspB1 constitutes a novel cytoprotective mechanism of proteostasis. Whether chaperone-mediated cytoprotective sequestration of toxic aggregates may bear clues to plaque deposition and may have potential therapeutic implications must be investigated in the future.
Figures
Fig. 1.
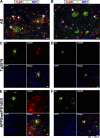
Localization of HspB1 in AD and transgenic mouse models of AD. Immunohistochemistry of HspB1 (A, C, and E) and HspB5 (B, D, and F) in AD hippocampal sections (A and B), cortical sections of 12-month-old Tg2576 mice (C and D), and 18-month-old APPswePS1dE9 mice (E and F) to show colocalization with plaques. Colocalization of Aβ (green) and HspB1 or HspB5 (red) with the nuclear stain DAPI (4′,6-diamidino-2-phenylindole; blue). The arrows in panels A and B indicate the reactive astrocytes. The asterisks in panel A indicate the penumbral staining of HspB1 (no penumbral staining was observed for HspB5).
Fig. 2.
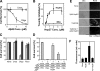
HspB1 abolishes toxicity of Aβ oligomers. (A) Dose-response curves for cytotoxicity of the Aβ conformers—unassembled (Unas) and oligomers (Oligo)—on N2a cells. Cell viability was monitored by CellTiter Blue assays. The x axis corresponds to monomer concentrations of Aβ. (B) Titration of HspB1 against Aβ oligomers. Cell viability values at various concentrations of monomeric HspB1 were plotted as the percentage of toxicity inhibited. Toxicity of Aβ oligomers (10 μM as monomer) in the absence of HspB1 was set to zero, and cell viability in buffer control was set to 100%. The values were fitted to a ligand binding equation with an _R_2 of 0.86. (C) N2a cells were treated with 10 μM Aβ conformers—unassembled (Unas), oligomers (Oligo), or fibrils (Fiber)—before (light gray bars) or after (dark gray bars) coincubation with purified human HspB1 (2 μM as monomer). Cells treated with buffer or HspB1 alone (None) had no influence on cell viability. (D) Effect of the order of addition of HspB1 on oligomer-mediated toxicity. The concentrations of all components were identical to those in panel B. The various treatments are numbered 1 to 5, and the treatments are listed in steps 1 to 3. Cells treated with buffer (treatment 1), oligomer (treatment 2), or oligomer coincubated with HspB1 (treatment 3) confirmed the results described in panel B. In treatment 4, cells were first treated with HspB1, followed by the addition of oligomer without washing off the HspB1. In treatment 5, cells were first treated with HspB1, which was washed away prior to oligomer addition. (E) Accumulation of reactive oxygen species (ROS) in N2a cells was detected using DCFDA fluorescence by microscopy. The left panels show differential interference contrast (DIC) images, and the right panels show fluorescence images (ROS). Incubation of N2a cells with Aβ oligomers (10 μM as monomer) alone resulted in the accumulation of ROS in nearly all cells. Treatment of cells with buffer or HspB1 (2 μM as monomer) alone did not induce ROS. Cells treated with oligomers preincubated with HspB1 did not accumulate ROS. (F) Quantitation of data shown in panel E by ImageJ software.
Fig. 3.
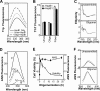
Characterization of the effect of HspB1 on Aβ conformers. (A) Interaction between Aβ conformers as indicated (10 μM as monomers) and HspB1 (2 μM as monomers) was monitored by the fluorescence properties of the tryptophans in HspB1. Fluorescence spectra were recorded between 310 and 370 nm after excitation at 295 nm in the absence of Aβ (HspB1 Only) or in the presence of unassembled Aβ (Unas) and oligomers (Oligo). (B) Effect of HspB1 on Aβ conformers was monitored by the amyloid-specific fluorescent dye thioflavin T (ThT; 20 μM). Excitation was at 420 nm, and emission was monitored at 450 to 550 nm. Fluorescence values at 484 nm of samples before (−HspB1) or after (+HspB1) coincubating Aβ conformers (10 μM as monomers) with HspB1 (2 μM as monomers) are plotted. (C) Secondary structural change in Aβ before (gray lines) or after (black lines) coincubation with HspB1 was monitored by circular dichroism spectroscopy between 190 and 250 nm. Ellipticity values shown are mean residue weight molar ellipticity (×1,000) with the unit degree·cm2·dmol−1. (D) Fluorescence studies using an extrinsic fluorophore, ANS, to detect conformational change in Aβ conformers. Aβ peptide was at 10 μM in all samples, and HspB1 was at 2 μM. ANS (10 μM) fluorescence was recorded between 420 and 570 nm after excitation at 370 nm. B, buffer; U, unassembled; O, oligomer; H, HspB1; F, fiber. (E) Kinetics of Aβ oligomer formation was monitored by ANS fluorescence change (y axis on right) and acquisition of cytotoxicity (Viability; y axis on left). (F) Conformational change in the Aβ (10 μM as monomers) and HspB1 (2 μM as monomers) coassemblies was monitored by ANS fluorescence. The actual fluorescence spectra (black lines) of unassembled and oligomer samples preincubated with HspB1 were compared with the expected algebraic sums (gray lines) of the spectra for Aβ and HspB1 collected separately.
Fig. 4.
Oligomers are converted to large aggregates by HspB1. (A) Atomic force microscopy images of unassembled Aβ and oligomers before (−HspB1) and after (+HspB1) coincubation with HspB1. Each field of view is 2 μm by 2 μm. The color bar on the right indicates the height of the particles. Bar, 500 nm. (B) The AFM images for oligomers without HspB1 and with HspB1 were analyzed for the height (blue) and area (red) of particles. (C) Electron microscopy of oligomers, oligomer coincubated with HspB1, and HspB1. The arrowheads in the middle panel indicate the large aggregates. (D) Kinetics of interaction between Aβ and HspB1 was monitored by light scatter. Light scatter of unassembled or oligomer samples of Aβ (10 μM as monomers) was examined at 350 nm (5-nm band-pass) in the absence (Buffer) or presence of 2 μM BSA or presence of 2 μM HspB1.
Fig. 5.
Participation of HspB1 in sequestering oligomers into aggregates. (A) Participation of HspB1 or BSA in aggregate formation was analyzed by centrifugation. Various concentrations (0.5, 1, or 2 μM as monomers) of HspB1 or BSA in the absence or presence of Aβ oligomers (10 μM as monomers) were centrifuged at 16,000 × g for 10 min. Uncentrifuged samples (Total), supernatants (Sup), and pellets (Pel) were analyzed by SDS-PAGE and stained with Coomassie blue. (B) Colocalization of Aβ and HspB1 in the aggregates. HspB1, Aβ oligomers, or oligomer-HspB1 aggregates (prepared as described for Fig. 2) were bound to polylysine-coated glass coverslips and probed with anti-Aβ (green) and anti-HspB1 (red). Bottom panels show merged images, with yellow showing colocalization. Bar, 80 μm.
Fig. 6.
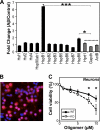
Importance of cellular HspB1 in Aβ toxicity. (A) Fold change in the expression of sHsps (7 out of 10 homologs, HspB1, -2, -3, -5, -6, -7, and -8), heat shock transcription factors (Hsf1, Hsf2, and Hsf4), Hsp90ab1, and housekeeping genes (Hprt1, GAPDH, and ActB) as monitored by reverse transcriptase PCR (RT-PCR). N2a cells were treated with Aβ oligomers (10 μM as monomers) for 1 h, after which Aβ was removed by replacing the medium. Cells were incubated in serum-rich medium for an additional 2 h, and RNA was extracted. As control, cells were treated identically but without Aβ addition. Fold changes in gene expression between Aβ-treated and control cells were plotted. Data were compared against those for the housekeeping genes by two-sample t test, and asterisks indicate statistically significant differences. (B) Representative preparation of primary mouse cortical neurons showing an overlay of staining for neuronal marker NeuN (red) and DAPI (blue). Magenta indicates neurons. (C) The toxicity of various concentrations of Aβ oligomers was tested on wild-type (WT) or HspB1-deficient (KO) neurons. Cell viability was measured by CellTiter Blue assay. Data were compared by two-sample t test, and asterisks indicate statistically significant differences.
Fig. 7.
Model for the sequestration of toxic oligomers into nontoxic aggregates. Assembly of misfolded proteins (e.g., Aβ) leads to the formation of toxic oligomers as one form of aggregates. Oligomers are thought to cause neuronal dysfunction and death central to neurodegenerative diseases. In this study, we have shown that HspB1 sequesters toxic oligomers into large nontoxic coaggregates and confers neuroprotection. The exact relationship between the large nontoxic aggregates containing HspB1 and Aβ oligomers observed in biochemical studies and HspB1 observed in pathological deposits is currently unknown.
Similar articles
- Specific sequences in the N-terminal domain of human small heat-shock protein HSPB6 dictate preferential hetero-oligomerization with the orthologue HSPB1.
Heirbaut M, Lermyte F, Martin EM, Beelen S, Sobott F, Strelkov SV, Weeks SD. Heirbaut M, et al. J Biol Chem. 2017 Jun 16;292(24):9944-9957. doi: 10.1074/jbc.M116.773515. Epub 2017 May 9. J Biol Chem. 2017. PMID: 28487364 Free PMC article. - Chaperone activity of human small heat shock protein-GST fusion proteins.
Arbach H, Butler C, McMenimen KA. Arbach H, et al. Cell Stress Chaperones. 2017 Jul;22(4):503-515. doi: 10.1007/s12192-017-0764-2. Epub 2017 Jan 27. Cell Stress Chaperones. 2017. PMID: 28130664 Free PMC article. - Human prefoldin inhibits amyloid-β (Aβ) fibrillation and contributes to formation of nontoxic Aβ aggregates.
Sörgjerd KM, Zako T, Sakono M, Stirling PC, Leroux MR, Saito T, Nilsson P, Sekimoto M, Saido TC, Maeda M. Sörgjerd KM, et al. Biochemistry. 2013 May 21;52(20):3532-42. doi: 10.1021/bi301705c. Epub 2013 May 8. Biochemistry. 2013. PMID: 23614719 - Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease.
Watson D, Castaño E, Kokjohn TA, Kuo YM, Lyubchenko Y, Pinsky D, Connolly ES Jr, Esh C, Luehrs DC, Stine WB, Rowse LM, Emmerling MR, Roher AE. Watson D, et al. Neurol Res. 2005 Dec;27(8):869-81. doi: 10.1179/016164105X49436. Neurol Res. 2005. PMID: 16354549 Review. - Peptide aptamers: tools to negatively or positively modulate HSPB1(27) function.
Gibert B, Simon S, Dimitrova V, Diaz-Latoud C, Arrigo AP. Gibert B, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Mar 25;368(1617):20120075. doi: 10.1098/rstb.2012.0075. Print 2013 May 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23530261 Free PMC article. Review.
Cited by
- Evolutionary Analyses and Natural Selection of Betaine-Homocysteine S-Methyltransferase (BHMT) and BHMT2 Genes.
Ganu RS, Ishida Y, Koutmos M, Kolokotronis SO, Roca AL, Garrow TA, Schook LB. Ganu RS, et al. PLoS One. 2015 Jul 27;10(7):e0134084. doi: 10.1371/journal.pone.0134084. eCollection 2015. PLoS One. 2015. PMID: 26213999 Free PMC article. - Truncation-Driven Lateral Association of α-Synuclein Hinders Amyloid Clearance by the Hsp70-Based Disaggregase.
Franco A, Cuéllar J, Fernández-Higuero JÁ, de la Arada I, Orozco N, Valpuesta JM, Prado A, Muga A. Franco A, et al. Int J Mol Sci. 2021 Nov 30;22(23):12983. doi: 10.3390/ijms222312983. Int J Mol Sci. 2021. PMID: 34884786 Free PMC article. - Integrating single-nucleus sequence profiling to reveal the transcriptional dynamics of Alzheimer's disease, Parkinson's disease, and multiple sclerosis.
Fan LY, Yang J, Liu RY, Kong Y, Guo GY, Xu YM. Fan LY, et al. J Transl Med. 2023 Sep 21;21(1):649. doi: 10.1186/s12967-023-04516-6. J Transl Med. 2023. PMID: 37735671 Free PMC article. - Therapeutic Strategies to Reduce the Toxicity of Misfolded Protein Oligomers.
Kreiser RP, Wright AK, Block NR, Hollows JE, Nguyen LT, LeForte K, Mannini B, Vendruscolo M, Limbocker R. Kreiser RP, et al. Int J Mol Sci. 2020 Nov 17;21(22):8651. doi: 10.3390/ijms21228651. Int J Mol Sci. 2020. PMID: 33212787 Free PMC article. Review. - Modeling the early stages of Alzheimer's disease by administering intracerebroventricular injections of human native Aβ oligomers to rats.
Baerends E, Soud K, Folke J, Pedersen AK, Henmar S, Konrad L, Lycas MD, Mori Y, Pakkenberg B, Woldbye DPD, Dmytriyeva O, Pankratova S. Baerends E, et al. Acta Neuropathol Commun. 2022 Aug 16;10(1):113. doi: 10.1186/s40478-022-01417-5. Acta Neuropathol Commun. 2022. PMID: 35974377 Free PMC article.
References
- Armstrong C. L., Krueger-Naug A. M., Currie R. W., Hawkes R. 2001. Constitutive expression of heat shock protein HSP25 in the central nervous system of the developing and adult mouse. J. Comp. Neurol. 434:262–274 - PubMed
- Arrigo A. P. 2007. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv. Exp. Med. Biol. 594:14–26 - PubMed
- Arrigo A. P., Landry J. 1994. Expression and function of the low-molecular-weight heat shock proteins, p. 335.In Morimoto R. I. (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Plainview, NY
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous