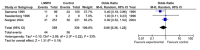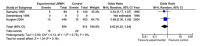Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery - PubMed (original) (raw)
Review
Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery
Alistair J Geraghty et al. Cochrane Database Syst Rev. 2011.
Abstract
Background: Peripheral arterial disease (PAD) is frequently treated by either an infrainguinal autologous (using the patient's own veins) or synthetic graft bypass. The rate of occlusion of the graft after one year is between 12% and 60%. To prevent occlusion, patients are treated with an antiplatelet or antithrombotic drug, or a combination of both. Little is known about which drug is optimal to prevent infrainguinal graft occlusion. This is an update of a Cochrane review first published in 2003.
Objectives: To evaluate whether antithrombotic treatment improves graft patency, limb salvage and survival in patients with chronic PAD undergoing infrainguinal bypass surgery.
Search strategy: The Cochrane Peripheral Vascular Diseases Group searched their Specialised Register (last searched August 2010) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3).
Selection criteria: Randomised, controlled trials; two review authors independently assessed the methodological quality of each trial using a standardised checklist.
Data collection and analysis: Data collected included patient details, inclusion and exclusion criteria, type of graft, antithrombotic therapy, outcomes, and side effects.
Main results: A total of 14 trials were included in this review; 4970 patient results were analysed. Four trials evaluating vitamin K antagonists (VKA) versus no VKA suggested that oral anticoagulation may favour autologous venous, but not artificial, graft patency as well as limb salvage and survival. Two other studies comparing VKA with aspirin (ASA) or aspirin and dipyridamole provided evidence to support a positive effect of VKA on the patency of venous but not artificial grafts. Three trials comparing low molecular weight heparin (LMWH) to unfractionated heparin (UFH) failed to demonstrate a significant difference on patency. One trial comparing LMWH with placebo found no significant improvement in graft patency over the first postoperative year in a population receiving aspirin. One trial showed an advantage for LMWH versus aspirin and dipyridamol at one year for patients undergoing limb salvage procedures. Perioperative administration of ancrod showed no greater benefit when compared to unfractionated heparin. Dextran 70 showed similar graft patency rates to LMWH but a significantly higher proportion of patients developed heart failure with dextran.
Authors' conclusions: Patients undergoing infrainguinal venous graft are more likely to benefit from treatment with VKA than platelet inhibitors. Patients receiving an artificial graft benefit from platelet inhibitors (aspirin). However, the evidence is not conclusive. Randomised controlled trials with larger patient numbers are needed in the future to compare antithrombotic therapies with either placebo or antiplatelet therapies.
Conflict of interest statement
None known
Figures
1
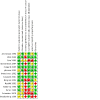
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3
CAPPA feedback April 2014: Patency at day 30
4
CAPPA feedback April 2014: Mortality at day 30
1.1. Analysis
Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Occlusion in all bypasses, 3 months
1.2. Analysis
Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Occlusion in all bypasses, 6 months
1.3. Analysis
Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Occlusion in all bypasses, 12 months
1.4. Analysis
Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Occlusion in all bypasses, 24 months
1.5. Analysis
Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Occlusion in all bypasses, 5 years
2.1. Analysis
Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb loss in all bypasses, 3 months
2.2. Analysis
Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Limb loss in all bypasses, 6 months
2.3. Analysis
Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Limb loss in all bypasses, 12 months
2.4. Analysis
Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Limb loss in all bypasses, 24 months
2.5. Analysis
Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Limb loss in all bypasses, 5 years
3.1. Analysis
Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Deaths in all bypasses, 3 months
3.2. Analysis
Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Deaths in all bypasses, 6 months
3.3. Analysis
Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Deaths in all bypasses, 12 months
3.4. Analysis
Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Deaths in all bypasses, 24 months
3.5. Analysis
Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Deaths in all bypasses, 5 years
4.1. Analysis
Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Occlusion in venous bypasses, 3 months
4.2. Analysis
Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Occlusion in venous bypasses, 6 months
4.3. Analysis
Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Occlusion in venous bypasses, 12 months
4.4. Analysis
Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Occlusion in venous bypasses, 24 months
4.5. Analysis
Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Occlusion in venous bypasses, 5 years
5.1. Analysis
Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb loss in venous bypasses, 3 months
5.2. Analysis
Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Limb loss in venous bypasses, 6 months
5.3. Analysis
Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Limb loss in venous bypasses, 12 months
5.4. Analysis
Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Limb loss in venous bypasses, 24 months
5.5. Analysis
Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Limb loss in venous bypasses, 5 years
6.1. Analysis
Comparison 6: Limb salvage venous grafts, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb salvage 6 months
7.1. Analysis
Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Deaths in venous bypasses, 3 months
7.2. Analysis
Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Deaths in venous bypasses, 6 months
7.3. Analysis
Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Deaths in venous bypasses, 12 months
7.4. Analysis
Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Deaths in venous bypasses, 24 months
7.5. Analysis
Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Deaths in venous bypasses, 5 years
8.1. Analysis
Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Occlusion in artificial bypasses, 3 months
8.2. Analysis
Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Occlusion in artificial bypasses, 6 months
8.3. Analysis
Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Occlusion in artificial bypasses, 12 months
8.4. Analysis
Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Occlusion in artificial bypasses, 24 months
8.5. Analysis
Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Occlusion in artificial bypasses, 5 years
9.1. Analysis
Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb loss in artificial bypasses, 3 months
9.2. Analysis
Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Limb loss in artificial bypasses, 6 months
9.3. Analysis
Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Limb loss in artificial bypasses, 12 months
9.4. Analysis
Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Limb loss in artificial bypasses, 24 months
9.5. Analysis
Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Limb loss in artificial bypasses, 5 years
10.1. Analysis
Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Deaths in artificial bypasses, 3 months
10.2. Analysis
Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Deaths in artificial bypasses, 6 months
10.3. Analysis
Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Deaths in artificial bypasses, 12 months
10.4. Analysis
Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Deaths in artificial bypasses, 24 months
10.5. Analysis
Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Deaths in artificial bypasses, 5 years
11.1. Analysis
Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 1: Occlusion in all bypasses, 3 months
11.2. Analysis
Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 2: Occlusion in all bypasses, 6 months
11.3. Analysis
Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 3: Occlusion in all bypasses, 12 months
11.4. Analysis
Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 4: Occlusion in all bypasses, 24 months
12.1. Analysis
Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 1: Occlusion in venous bypasses, 3 months
12.2. Analysis
Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 2: Occlusion in venous bypasses, 6 months
12.3. Analysis
Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 3: Occlusion in venous bypasses, 12 months
12.4. Analysis
Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 4: Occlusion in venous bypasses, 24 months
13.1. Analysis
Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 1: Occlusion in non‐venous bypasses, 3 months
13.2. Analysis
Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 2: Occlusion in non‐venous bypasses, 6 months
13.3. Analysis
Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 3: Occlusion in non‐venous bypasses, 12 months
13.4. Analysis
Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 4: Occlusion in non‐venous bypasses, 24 months
14.1. Analysis
Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1: Occlusion in all bypasses, 24 hours
14.2. Analysis
Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2: Occlusion in all bypasses, day 10
14.3. Analysis
Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3: Occlusion,in all bypasses, day 30
14.4. Analysis
Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 4: Per protocol
15.1. Analysis
Comparison 15: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus ASA/DIP, Outcome 1: Occlusion in all bypasses, 6 months
15.2. Analysis
Comparison 15: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus ASA/DIP, Outcome 2: Occlusion in all bypasses, 12 months
16.1. Analysis
Comparison 16: Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH, Outcome 1: Occlusion in all bypasses, 1 month
16.2. Analysis
Comparison 16: Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH, Outcome 2: Occlusion in all bypasses, 3 months
16.3. Analysis
Comparison 16: Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH, Outcome 3: Occlusion in all bypasses, 12 months
17.1. Analysis
Comparison 17: Survival venous grafts, Sarac study, Outcome 1: Survival 6 months, intention to treat
17.2. Analysis
Comparison 17: Survival venous grafts, Sarac study, Outcome 2: Survival 2 years, intention to treat
18.1. Analysis
Comparison 18: Early graft thrombosis, ancrod versus heparin, Outcome 1: Early graft thrombosis, 24 h and 1 month
19.1. Analysis
Comparison 19: Early graft thrombosis, unfractionated heparin (UFH) versus antithrombin, Outcome 1: Antithrombin versus UFH, intraoperative graft thrombosis
19.2. Analysis
Comparison 19: Early graft thrombosis, unfractionated heparin (UFH) versus antithrombin, Outcome 2: Antithrombin versus UFH, 1 month occlusion (following thrombendarterectomy)
20.1. Analysis
Comparison 20: Early graft occlusion, low molecular weight heparin (LMWH) versus dextran, Outcome 1: Early graft occlusion, 30 days
Update of
- Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery.
Dörffler-Melly J, Büller HR, Koopman MM, Prins MH. Dörffler-Melly J, et al. Cochrane Database Syst Rev. 2003;(4):CD000536. doi: 10.1002/14651858.CD000536. Cochrane Database Syst Rev. 2003. PMID: 14583924 Updated. Review.
Comment in
- Evidence from Cochrane systematic reviews for effects of antithrombotic drugs for lower-limb revascularization. A narrative review.
Dias SVM, Flumignan RLG, Iared W. Dias SVM, et al. Sao Paulo Med J. 2021 Aug 13;139(6):675-684. doi: 10.1590/1516-3180.2020.0640.110321. eCollection 2021. Sao Paulo Med J. 2021. PMID: 34406310 Free PMC article.
Similar articles
- Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery.
Dörffler-Melly J, Büller HR, Koopman MM, Prins MH. Dörffler-Melly J, et al. Cochrane Database Syst Rev. 2003;(4):CD000536. doi: 10.1002/14651858.CD000536. Cochrane Database Syst Rev. 2003. PMID: 14583924 Updated. Review. - Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery.
Bedenis R, Lethaby A, Maxwell H, Acosta S, Prins MH. Bedenis R, et al. Cochrane Database Syst Rev. 2015 Feb 19;2015(2):CD000535. doi: 10.1002/14651858.CD000535.pub3. Cochrane Database Syst Rev. 2015. PMID: 25695213 Free PMC article. Review. - Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery.
Brown J, Lethaby A, Maxwell H, Wawrzyniak AJ, Prins MH. Brown J, et al. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD000535. doi: 10.1002/14651858.CD000535.pub2. Cochrane Database Syst Rev. 2008. PMID: 18843613 Updated. Review. - Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery.
Dörffler-Melly J, Koopman MM, Adam DJ, Büller HR, Prins MH. Dörffler-Melly J, et al. Cochrane Database Syst Rev. 2003;(3):CD000535. doi: 10.1002/14651858.CD000535. Cochrane Database Syst Rev. 2003. PMID: 12917893 Updated. Review. - Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment.
Robertson L, Ghouri MA, Kovacs F. Robertson L, et al. Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD002071. doi: 10.1002/14651858.CD002071.pub3. Cochrane Database Syst Rev. 2012. PMID: 22895926 Free PMC article. Review.
Cited by
- Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia.
Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, Aboyans V, Aksoy M, Alexandrescu VA, Armstrong D, Azuma N, Belch J, Bergoeing M, Bjorck M, Chakfé N, Cheng S, Dawson J, Debus ES, Dueck A, Duval S, Eckstein HH, Ferraresi R, Gambhir R, Gargiulo M, Geraghty P, Goode S, Gray B, Guo W, Gupta PC, Hinchliffe R, Jetty P, Komori K, Lavery L, Liang W, Lookstein R, Menard M, Misra S, Miyata T, Moneta G, Munoa Prado JA, Munoz A, Paolini JE, Patel M, Pomposelli F, Powell R, Robless P, Rogers L, Schanzer A, Schneider P, Taylor S, De Ceniga MV, Veller M, Vermassen F, Wang J, Wang S; GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and World Federation of Vascular Societies (WFVS). Conte MS, et al. Eur J Vasc Endovasc Surg. 2019 Jul;58(1S):S1-S109.e33. doi: 10.1016/j.ejvs.2019.05.006. Epub 2019 Jun 8. Eur J Vasc Endovasc Surg. 2019. PMID: 31182334 Free PMC article. - Intermittent claudication: new targets for drug development.
Brass EP. Brass EP. Drugs. 2013 Jul;73(10):999-1014. doi: 10.1007/s40265-013-0078-3. Drugs. 2013. PMID: 23775528 Review. - A review of the role of anticoagulation in the treatment of peripheral arterial disease.
Whayne TF. Whayne TF. Int J Angiol. 2012 Dec;21(4):187-94. doi: 10.1055/s-0032-1330232. Int J Angiol. 2012. PMID: 24293975 Free PMC article. Review. - Evidence from Cochrane systematic reviews for effects of antithrombotic drugs for lower-limb revascularization. A narrative review.
Dias SVM, Flumignan RLG, Iared W. Dias SVM, et al. Sao Paulo Med J. 2021 Aug 13;139(6):675-684. doi: 10.1590/1516-3180.2020.0640.110321. eCollection 2021. Sao Paulo Med J. 2021. PMID: 34406310 Free PMC article. - Tibial bypass salvage with eptifibatide in a patient with thrombocythemia.
Byrne J, Greco E, Yeo E, McCluskey S, Lindsay T. Byrne J, et al. J Vasc Surg Cases. 2015 Nov 1;1(4):246-248. doi: 10.1016/j.jvsc.2015.08.004. eCollection 2015 Dec. J Vasc Surg Cases. 2015. PMID: 31724642 Free PMC article.
References
References to studies included in this review
Arfvidsson 1990 {published data only}
- Arfvidsson B, Lundgren F, Drott C, Schersten T, Lundholm K. Influence of coumarin treatment on patency and limb salvage after peripheral arterial reconstructive surgery. American Journal of Surgery 1990;159(6):556-60. - PubMed
BOA 2000 {published data only}
- Algra A. A randomised trial of oral anticoagulants versus aspirin after infrainguinal bypass surgery. Tijdschrift voor Sociale Gezondheidszorg 1999;77(4):11.
- Ariesen MJ, Tangelder MJ, Lawson JA, Eikelboom BC, Grobbee DE, Algra A, et al. Risk of major haemorrhage in patients after infrainguinal venous bypass surgery: therapeutic consequences? The Dutch BOA (Bypass Oral Anticoagulants or Aspirin) study. European Journal of Vascular and Endovascular Surgery 2005;30(2):154-9. - PubMed
- Dutch Bypass Oral anticoagulants of Aspirin (BOA) Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. Lancet 2000;355(9201):346-51. - PubMed
- Eikelboom BC. Prevention of occlusions after infrainguinal bypass surgery with oral anticoagulants or acetylsalicylic acid: A randomised trial. Nederlands Tijdschrift voor Geneeskunde 2001;145(22):1060-7. - PubMed
- Gorter JW, Oostenbrink JB, Tangelder MJ, Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group, Dutch Bypass Oral, et al. Costs of outpatient anticoagulant treatment in patients with cerebral and peripheral arterial occlusive disease. Thrombosis and Haemostasis 2001;85(1):52-6. - PubMed
Cole 1993 {published data only}
- Cole CW, Bormanis J, Luna GK, Hajjar G, Barber GG, Harris KA et al. Ancrod versus heparin for anticoagulation during vascular surgical procedures. Journal of Vascular Surgery 1993;17(2):288-93. - PubMed
Edmondson 1994 {published data only}
- Edmondson RA, Cohen AT, Das SK, Wagner MB, Kakkar VV. Low-molecular weight heparin versus aspirin and dipyridamole after femoropopliteal bypass grafting. Lancet 1994;344:914-8. - PubMed
- Kakkar VV, Edmondson RA, Cohen AJ, Phillips MJ. A prospective, randomized trial of low molecular weight heparin (LMWH) versus persantin in femoropopliteal bypass grafts. Thrombosis and Haemostasis 1993;69(6):647-Abstract No 375.
Jivegard 2005 {published and unpublished data}
- Jivegard L, Drott C, Gelin J, Groth O, Hensater M, Jensen N, et al. Effects of three months of low molecular weight heparin (dalteparin) treatment after bypass surgery for lower limb ischemia - A randomised placebo-controlled double blind multicentre trial. European Journal of Vascular and Endovascular Surgery 2005;29(2):190-8. - PubMed
Johnson 2002 {published data only}
- Jackson MR, Johnson WC, Williford WO, Valentine RJ, Clagett GP. The effect of anticoagulation therapy and graft selection on the ischemic consequences of femoropopliteal bypass graft occlusion: results from a multicenter randomized clinical trial. Journal of Vascular Surgery 2002;35(2):292-8. - PubMed
- Johnson WC, Williford WO, Corson JD, Padberg FT Jr. Hemorrhagic complications during long-term postoperative warfarin administration in patients undergoing lower extremity arterial bypass surgery. Vascular 2004;12(6):362-8. - PubMed
- Johnson WC, Williford WO and members of the Department of Veterans Affairs Cooperative Study. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: A prospective randomized study. Journal of Vascular Surgery 2002;35(3):413-21. - PubMed
- Kibbe MR, Hassett AL, McSherry F, Conner P, Bontempo FA, Williford W, et al. Can screening for genetic markers improve peripheral artery bypass patency? Journal of Vascular Surgery 2002;36(6):1198-206. - PubMed
Kretschmer 1992 {published data only}
- Kretschmer G, Herbst F, Prager M, Sautner T, Wenzl E, Berlakovich GA, et al. A decade of oral anticoagulants treatment to maintain autologous vein grafts for femoropopliteal atherosclerosis. Archives of Surgery 1992;127:1112-5. - PubMed
- Kretschmer G, Wenzl E, Wagner O, Polterauer P, Piza F, Schemper M, et al. Can anticoagulants prevent graft reocclusion? In: Maurer PC, Becker HM, Heidrich H, Hoffmann G, Kriessmann A, Muller-Wiefel H, Pratorius C, editors(s). What is new in Angiology?. Zuckschwerdt W, 1986:200-1.
Logason 2001 {published data only}
- Logason K, Bergqvist D, Study Group on AntIthrombotic Prophylaxis of Femorodistal Bypass Surgery. Low molecular weight heparin (enoxaparin) versus dextran in the prevention of early occlusion following arterial bypass surgery distal to the groin. European Journal of Vascular and Endovascular Surgery 2001;21(3):261-5. - PubMed
- Logason K. Low-molecular weight heparin versus dextran in the prevention of early occlusion following arterial bypass surgery distal to the groin. European Journal of Surgery, Supplement 1998;(581):40-1. - PubMed
Norgren 2004 {published data only}
- Norgren L, Swedish EnoxaVasc Study Group. Can low molecular weight heparin replace unfractionated heparin during peripheral arterial reconstruction? An open label prospective randomized controlled trial. Journal of Vascular Surgery 2004;39(5):977-84. - PubMed
Nydahl 1992 {published data only}
- Nydahl S, Swedenborg J, Egberg N. Peroperative anticoagulation with antithrombin or heparin in infrainguinal bypass surgery. European Journal of Vascular Surgery 1992;6:610-5. - PubMed
Samama 1994 {published data only}
- Samama CM, Gigou F, Ill P. Low-molecular weight heparin vs. unfractionated heparin in femorodistal reconstructive surgery: a multicenter open randomized study. Annals of Vascular Surgery 1995;9 Suppl:45-53. - PubMed
- Samama CM. Low molecular weight heparin (enoxaparin) versus unfractionated heparin during and after arterial reconstructive surgery; A multicenter randomized study. Thrombosis and Haemostasis 1993;69(6):546-Abstract No 24.
Sarac 1998 {published data only}
- Sarac TP, Huber TS, Back MR, Ozaki CK, Carlton LM, Flynn TC, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. Journal of Vascular Surgery 1998;28(3):446-517. - PubMed
Schneider 1979 {published data only}
- Bollinger A, Schneider E, Pouliadis G, Torres C, Brunner U. Antiplatelet drugs and anticoagulants after reconstructive surgery of the femoro-popliteal arteries. Results of a prospective study. In: Prophylaxis of Venous, Peripheral, Cardiac and Cerebral Vascular Diseases with Acetylsalicylic Acid. Stuttgart, New York: F.K. Schattauer Verlag, 1980:73-8.
- Schneider E, Brunner U, Bollinger A. Medical treatment for preventing recurrence after femoro-popliteal arterial reconstruction [Medikamentose Rezidivprophylaxe nach femoro-poplitealer Arterienrekonstruktion]. Angio 1979;2(1):73-7.
Swedenborg 1996 {published data only}
- Swedenborg J, Nydahl S, Egberg N. Low molecular mass heparin can be used as peri-operative anticoagulant during infrainguinal bypass surgery. Thrombosis and Haemostasis 1995;73(6):979-Abstract No 308.
- Swedenborg J, Nydahl S, Egberg N. Low molecular mass heparin instead of unfractionated heparin during infrainguinal bypass surgery. European Journal of Vascular and Endovascular Surgery 1996;11(1):59-64. - PubMed
References to studies excluded from this review
Alja‐Kulju 1990 {published data only}
- Alja-Kulju K, Ketonen P, Salo J, Sipponen J, Verkkala, K, Harjola P-T. Effect of antiplatelet and anticoagulant therapy on patency of femorotibial bypass grafts. Journal of Cardiovascular Surgery 1990;31(5):651-5. - PubMed
Benedetti 1988 {published data only}
- Benedetti-Valentini F, Irace L, Gattuso R, Ciocca F, Aracu A, Intrieri F, et al. Arterial repair of the lower limbs: prevention of prosthetic grafts occlusion by LMW-heparin. International Angiology 1988;7 Suppl 3:29-32. - PubMed
Evans 1966 {published data only}
- Evans G, Irvine WT. Long-term arterial-graft patency in relation to platelet adhesiveness, biochemical factors, and anticoagulant therapy. Lancet 1966;2(7459):353-5. - PubMed
Fietze‐Fischer 1987 {published data only}
- Fietze-Fischer B, Gruss JD, Bartels D, et al. Prostaglandin E1 as an adjuvant therapy in the event of femoropopliteal and crural great saphenous vein in situ bypass surgery. Vasa 1987;16 Suppl 17:23-5. - PubMed
- Fietze-Fischer B, Gruss JD, Bartels D, Vargas-Montano H, Stritter W. Prostaglandin E1 as an adjuvant therapy in the event of femoropopliteal and crural great saphenous vein in situ bypass surgery. Vasa 1987;16 Suppl 17:23-5. - PubMed
Gruss 1991 {published data only}
- Gruss JD, Hiemer W, Kroiss A, Geissler C. Experience with adjuvant prostaglandin therapy in vascular surgery. Vasa 1991;20 Suppl 33:353-4. - PubMed
Kretschmer 1987 {published data only}
- Kretschmer G, Wenzl E, Piza F, Polterauer P, Ehringer H, Minar E, et al. The influence of anticoagulant treatment on the probability of function in femoropopliteal vein bypass surgery: Analysis of a clinical series (1970 to 1985) and interim evaluation of a controlled clinical trial. Surgery 1987;102(3):453-9. - PubMed
Kretschmer 1988 {published data only}
- Kretschmer G, Wenzl E, Schemper M, Huk I, Konecny U, Marosi L et al. Vein bypass surgery for femoro-popliteal ateriosclerosis: influence of different risk factors on patient survival and the importance of anticoagulant treatment. European Journal of Vascular Surgery 1988;2(2):77-81. - PubMed
Kujath 2002 {published data only}
- Kujath P, Eckmann C, Misselwitz F. Low-molecular-weight heparin in arterial reconstructive surgery: a double-blind, randomized dose-finding trial. Clinical and Applied Thrombosis/Hemostasis 2002;8(4):337-45. - PubMed
McMillan 1997 {published data only}
- McMillan WD, McCarthy WJ, Lin SJ, Matsumura JS, Pearce WH, Yao JST. Perioperative low molecular weight heparin for infrageniculate bypass. Journal of Vascular Surgery 1997;25(5):796-801. - PubMed
Ohtake 1997 {published data only}
- Ohtake H, Urayama H, Kimura, K, Yokoi K, Tsunezuka Y, Kawakami T, et al. Comparison of cilostazol with warfarin as antithrombotic therapy after femoro-popliteal bypass surgery using an e-PTFE graft. Minerva Cardioangiologica 1997;45(11):527-30. - PubMed
Ray 1997 {published data only}
- Ray SA, Rowley MR, Bevan DH, Taylor RS, Dormandy JA. Hypercoagulable abnormalities and postoperative failure of arterial reconstruction. European Journal of Endovascular Surgery 1997;13(4):363-70. - PubMed
Rosenthal 1987 {published data only}
- Rosenthal D, Mittenthal MH, Ruben DM, Jones DH, Estes JW, Stanton PE, et al. The effects of aspirin, dipyridamole and warfarin in femorodistal reconstructions: long-term results. American Surgeon 1987;53(9):477-81. - PubMed
Waibel 1981 {published data only}
- Waibel P, Geering P. Late results of anticoagulation in arterial reconstructions. Vasa 1981;10:308-9. - PubMed
Additional references
Abbott 1997
- Abbott WM, Green RM, Matsumoto T, Wheeler JR, Miller N, Veith FJ, et al. Prosthetic above-knee femoropopliteal bypass grafting: results of a multicenter randomized prospective trial. Above-Knee Femoropopliteal Study Group. Journal of Vascular Surgery 1997;25(1):19-28. - PubMed
Achermann 1998
- Achermann A, Gurke L, Stirnemann P. Supragenicular bypass: venous in comparison with synthetic prosthesis (PTFE). Swiss Surgeon 1998;4(3):129-32. - PubMed
Alback 1998
- Alback A, Lepantalo M. Immediate occlusion of in situ saphenous vein bypass grafts: a survey of 329 reconstructions. European Journal of Surgery 1998;164(10):745-50. - PubMed
Bandyk 1987
- Bandyk DF, Kaebnick HW, Stewart GW, Towne JB. Durability of the in situ saphenous vein arterial bypass: a comparison of primary and secondary patency. Journal of Vascular Surgery 1987;5(2):256-68. - PubMed
CASPAR 2010
- Belch JJ, Dormandy J, Biasi BM, Cairols M, Diehm C, Eikelboom B, et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. Journal of Vascular Surgery 2010;52:825-33, 833. - PubMed
Channon 1997
- Channon KM, Fulton GJ, Davies MG, Peters KG, Ezekowitz MD, Hagen PO, et al. Modulation of tissue factor protein expression in experimental venous bypass grafts. Arteriosclerosis, Thrombosis, and Vascular Biology 1997;17(7):1313-9. - PubMed
Cochrane 2008
Cochrane 2010
Consensus 1991
- Anonymous. Second European Consensus Document on chronic critical leg ischaemia. Circulation 1991;84 Suppl 4:1-26. - PubMed
Davies 1994
- Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. The British Journal of Surgery 1994;81(9):1254-69. [PMID: ] - PubMed
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499-533. - PubMed
Girolami 2000
- Girolami B, Bernardi E, Prins MH, ten Cate JW, Prandoni P, Simioni P, et al. Antiplatelet therapy and other interventions after revascularisation procedures in patients with peripheral arterial disease: a meta-analysis. European Journal of Vascular and Endovascular Surgery 2000;19(4):370-80. - PubMed
Hanson 1987
- Hanson SR, Harker LA. Vascular graft thrombus formation. Annals of the New York Acadamy of Science 1987;516:653-61. - PubMed
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org.
Jacot 2004
- Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, et al. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. Journal of Vascular Surgery 2004;39:547-55. - PubMed
Johnson 2000
- Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. Journal of Vascular Surgery 2000;32(2):268-77. [PMID: ] - PubMed
Kissin 2000
- Kissin M, Kansal N, Pappas PJ, DeFouw DO, Duran WN, Hobson RW. Vein interposition cuffs decrease the intimal hyperplastic response of polytetrafluoroethylene bypass grafts. Journal of Vascular Surgery 2000;31:69-83. - PubMed
Kraiss 1995
- Kraiss LW, Johansen K. Pharmacologic intervention to prevent graft failure. Surgical Clinics of North America 1995;75(4):761-72. - PubMed
Kretschmer 1999
- Kretschmer G, Holzenbein TH. Oral anticoagulation in peripheral vascular surgery: how intense, for how long, or at all? Journal of Internal Medicine 1999;245(4):389-97. - PubMed
Lindblad 1995
- Lindblad B, Wakefield TW, Stanley TJ, Bergqvist D, Nichol BJ, Greenfield LJ, et al. Pharmacological prophylaxis against postoperative graft occlusion after peripheral vascular surgery: a world-wide survey. European Journal of Vascular and Endovascular Surgery 1995;9(3):267-71. - PubMed
Muluk 1998
- Muluk SC, Vorp DA, Severyn DA, Gleixner S, Johnson PC, Webster MW. Enhancement of tissue factor expression by vein segments exposed to coronary arterial hemodynamics. Journal of Vascular Surgery 1998;27(3):521-7. - PubMed
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery 2007;45 Suppl S:5-67. [PMID: ] - PubMed
Owens 2010
Parsons 1996
- Parsons RE, Suggs WD, Veith FJ, Sanchez LA, Lyon RT, Marin ML, et al. Polytetrafluoroethylene bypasses to infrapopliteal arteries without cuffs or patches: a better option than amputation in patients without autologous vein. Journal of Vascular Surgery 1996;23(2):347-54. - PubMed
Robinson 1997
- Robinson KD, Sato DT, Gregory RT, Gayle RG, DeMasi RJ, Parent FN 3rd. Long-term outcome after early infrainguinal graft failure. Journal of Vascular Surgery 1997;26(3):425-37. - PubMed
Sarkar 2006
- Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. European Journal of Vascular and Endovascular Surgery 2006;31:627-36. - PubMed
Sasaki 2000
Schoder 2001
- Schoder M, Cejna M, Lammer J. Interventions in infrainguinal bypass grafts. ROFO-Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren 2001;173(12):1059-68. - PubMed
Stept 1987
- Stept LL, Flinn WR, McCarthy WJ III, Bartlett ST, Bergan JJ, Yao JS. Technical defects as a cause of early graft failure after femorodistal bypass. Archives of Surgery 1987;122(5):599-604. - PubMed
Tangelder 1999
- Tangelder MJD, Lawson JA, Algra A, Eikelboom BC. Systematic review of randomized controlled trials of aspirin and oral anticoagulants in the prevention of graft occlusion and ischemic events after infrainguinal bypass surgery. Journal of Vascular Surgery 1999;30(4):701-9. - PubMed
Watson 1999
- Watson HR, Belcher G, Horrocks M. Adjuvant medical therapy in peripheral bypass surgery. The British Journal of Surgery 1999;86(8):981-91. - PubMed
Woodburn 1996
- Woodburn KR, Rumley A, Lowe GD, Love JG, Murray GD, Pollock JG. Clinical, biochemical, and rheological factors affecting the outcome of infrainguinal bypass grafting. Journal of Vascular Surgery 1996;24(4):639-46. - PubMed
References to other published versions of this review
Cochrane 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical