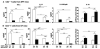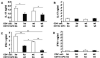Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice - PubMed (original) (raw)
Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice
Bo Liu et al. Gastroenterology. 2011 Aug.
Abstract
Background & aims: Mice that are deficient in interleukin (IL)-10 develop colitis, mediated by T-helper (Th)1 and Th17 cells, and IL-10-producing regulatory T (Treg) cells suppress colitis, implicating IL-10 in maintaining mucosal homeostasis. We assessed the relative importance of immunoregulatory IL-10 derived from T cells or from antigen presenting cells (APCs) in development of intestinal inflammation.
Methods: CD4(+) cells from germ-free (GF) or specific pathogen-free (SPF) IL-10(-/-) or wild-type mice were injected into IL-10(-/-), Rag2(-/-) mice or Rag2(-/-) mice that express IL-10. After 6-8 weeks, we evaluated inflammation, spontaneous secretion of cytokines from colonic tissue, and mRNA levels of the transcription factor T-bet and the immunoregulatory cytokine transforming growth factor (TGF)-β. CD4(+) T cells were co-cultured with bacterial lysate-pulsed APCs and assayed for cytokine production, FoxP3 expression, and TGF-β-mediated Smad signaling.
Results: CD4(+) cells from GF or SPF IL-10(-/-) or wild-type mice induced more severe colitis and higher levels of inflammatory cytokines in IL-10(-/-), Rag2(-/-) mice than in IL-10-replete, Rag2(-/-) mice. Co-cultures of IL-10(-/-) or wild-type CD4(+) T cells plus bacterial lysate-pulsed APCs from IL-10(-/-) mice contained more interferon (IFN)-γ, IL-12/23p40, and IL-17 than co-cultures of the same T cells plus APCs from wild-type mice. CD11b(+) APCs were required for these effects. Blocking IL-10 receptors increased production of IFN-γ and IL-12/23p40 whereas exogenous IL-10 suppressed these cytokines. IL-10-producing APCs induced TGF-β-mediated, retinoic acid-dependent, differentiation of FoxP3(+) Treg cells, whereas blocking the retinoic acid receptor, in vitro and in vivo, reduced proportions of FoxP3(+) Treg cells.
Conclusions: IL-10 produced by APCs regulates homeostatic T-cell responses to commensal bacteria.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1. Colonic inflammation in IL-10 wt Rag2−/− or IL-10 ko Rag2−/− recipients reconstituted with either SPF or GF IL-10 ko or wt CD4+ cells
(A) Representative H&E stained sections of the distal colons of recipient mice 6-8 wk after transfer of SPF CD4+ cells (20× magnification). (B) Blinded histologic scores in the large intestine and (C) spontaneous secretion of IFN-γ by colonic fragments of recipients of SPF CD4+ cells. (D) Representative H&E stained sections of the distal colons of recipient mice 6-8 wk after transfer of GF CD4+ cells. (E) Blinded histologic scores and (F) IFN-γ secretion by colonic fragments of recipients of GF CD4+ cells. Results show mean ± SEM, pooled from three separate experiments, with (ko→ko, n=14; ko→wt, n=12; wt→ko, n=11; wt→wt, n=11) for recipients of SPF CD4+ cells and (ko→ko, n=7; ko→wt, n=12; wt→ko, n=11; wt→wt, n=14) for recipients of GF CD4+ cells. *p< 0.05, **p<0.01 & ***p<0.001.
Figure 2. Cytokines produced by MLN cells after transfer of SPF or GF IL-10 ko or IL-10 wt CD4+ T cells
Six-eight weeks after transfer of either SPF (A) or GF (B) CD4+ cells, unseparated MLN cells from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipients were stimulated with CBL for 72 hours and IFN-γ, IL-17 and IL-12/23p40 secretion were measured. Spontaneous IL-10 by colonic fragments was evaluated. Results show mean ± SEM (number of mice/group same as Figure 1). *p< 0.05, **p<0.01, ***p<0.001.
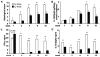
Figure 3. Time course of histological evaluation of colitis and proinflammatory cytokine production in recipient mice
(A) Histologic score, (B) spontaneous IL-12/23p40 in colonic cultures, and amounts of (C) IFN-γ and (D) IL-12/23p40 produced by unfractionated MLN cells stimulated with CBL (10 μg/ml) after 3 day culture in vitro for mice evaluated 1, 2, 4, 6 and 12 weeks after transfer of SPF IL-10 ko CD4+ T cells to IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipients. ** p< 0.001 and *p<0.05 or p value shown above the bar vs. IL-10 wt Rag2−/− recipients. Results show mean ± SEM, 6-8 mice per group at each time point.
Figure 4. In vitro cytokine production
Splenic CD4+ T cells from SPF IL-10 ko or IL-10 wt mice were co-cultured with CBL-pulsed APC from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− mice for 72 hours and (A) IFN-γ (B) IL-17, (C) IL-12/23p40 (D) IL-10 concentrations were measured. Representative results (one of three separate experiments) are shown as mean ± SEM of triplicate culture supernatants. ***p<0.001 vs. co-cultures containing IL-10 wt APC. §§§p<0.001 vs co-cultures containing IL-10 ko APC. □p<0.001 vs IL-10 wt APC alone or in co-culture with IL-10 ko CD4+ cells.
Figure 5. CD11b-enriched APC regulate cytokine production
APC from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− mice were separated into CD11b-enriched (CD11b+) or CD11b-depleted (CD11b−) populations by positive or negative magnetic bead sorting, respectively. Co-cultures contain either IL-10 ko or IL-10 wt splenic CD4+ T cells and CBL-pulsed APC. (A, B) IL-17 or (C, D) IFN-γ was measured in supernatants collected 72 hours after co-culture initiation. Representative results (one of three separate experiments) are shown as mean ± SEM of triplicate culture supernatants. *p< 0.05, **p<0.01.
Figure 6. Induction of FoxP3+ Treg cells by IL-10 wt Rag2−/− or IL-10 ko Rag2−/− APC
(A) Intracellular FoxP3 expressed by IL-10 wt CD4+ cells co-cultured with IL-10 wt or IL-10 ko Rag2−/− APC for 4 days in the presence or absence of TGF-β1 plus LE540 or vehicle (DMSO). (B) Intracellular FoxP3 in CD4+ cells from recipients treated in vivo with LE540 or vehicle (DMSO in soybean oil). (C) Expression of Aldh1a1 and Aldh1a2 mRNA relative to β-actin in MLN from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipient mice. Representative results (one of two separate experiments) are shown as mean ± SEM of triplicate qPCR. *p< 0.05, **p<0.01. (D) FoxP3 expression by IL-10 wt CD4+ cells co-cultured with IL-10 wt APC in the presence of TGF-β1(1 ng/ml) and anti-IL-10R or isotype control (30 μg/ml). (E) FoxP3 expression by IL-10 wt CD4+ cells co-cultured with IL-10 ko APC in the presence of TGF-β1 (1 ng/ml) with or without recombinant IL-10 (500 pg/ml) for 4 days. Representative results (one of three experiments) are shown for D and E.
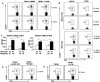
Figure 7. Smad3 and P-Smad3 protein and TGF-β1 mRNA expression
APC from IL-10 wt Rag2−/− and IL-10 ko Rag2−/− mice co-cultured with IL-10 wt CD4+ cells were stimulated with TGF-β1 in the presence of CBL (10 μg/ml). Cells were harvested at times shown and Western blot analysis visualized Smad3 and P-Smad3. (A) Representative blots from two experiments are shown. (B) Densitometric analysis of the Western blot shown in (A) p-Smad3/actin left panel, p-Smad3/total Smad3 right panel. (C) TGF-β1 mRNA expression in MLN from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipient mice reconstituted with IL-10 ko or IL-10 wt CD4+ cells. Representative results (one of three experiments) are shown as mean ± SEM of triplicate qPCR. (D) TGF-β1 mRNA expression in co-cultured IL-10 wt CD4+ cells with CBL-pulsed APC from either IL-10 ko Rag2−/− or IL-10 wt Rag2−/− mice. Representative results (one of two separate experiments) are shown as mean ± SEM of triplicate qPCR.
Similar articles
- Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis.
Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Feng T, et al. Gastroenterology. 2011 Jun;140(7):2031-43. doi: 10.1053/j.gastro.2011.03.009. Epub 2011 Mar 17. Gastroenterology. 2011. PMID: 21419767 Free PMC article. - Endogenous antigen presenting cell-derived IL-10 inhibits T lymphocyte responses to commensal enteric bacteria.
Albright CA, Sartor RB, Tonkonogy SL. Albright CA, et al. Immunol Lett. 2009 Mar 24;123(1):77-87. doi: 10.1016/j.imlet.2009.02.010. Epub 2009 Mar 3. Immunol Lett. 2009. PMID: 19428554 Free PMC article. - Microflora reactive IL-10 producing regulatory T cells are present in the colon of IL-2 deficient mice but lack efficacious inhibition of IFN-gamma and TNF-alpha production.
Waidmann M, Allemand Y, Lehmann J, di Genaro S, Bücheler N, Hamann A, Autenrieth IB. Waidmann M, et al. Gut. 2002 Feb;50(2):170-9. doi: 10.1136/gut.50.2.170. Gut. 2002. PMID: 11788555 Free PMC article. - IFN-γ and IL-17: the two faces of T-cell pathology in giant cell arteritis.
Weyand CM, Younge BR, Goronzy JJ. Weyand CM, et al. Curr Opin Rheumatol. 2011 Jan;23(1):43-9. doi: 10.1097/BOR.0b013e32833ee946. Curr Opin Rheumatol. 2011. PMID: 20827207 Free PMC article. Review. - Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells.
Pandiyan P, Zhu J. Pandiyan P, et al. Cytokine. 2015 Nov;76(1):13-24. doi: 10.1016/j.cyto.2015.07.005. Epub 2015 Jul 10. Cytokine. 2015. PMID: 26165923 Free PMC article. Review.
Cited by
- IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy.
Schmitt EG, Haribhai D, Williams JB, Aggarwal P, Jia S, Charbonnier LM, Yan K, Lorier R, Turner A, Ziegelbauer J, Georgiev P, Simpson P, Salzman NH, Hessner MJ, Broeckel U, Chatila TA, Williams CB. Schmitt EG, et al. J Immunol. 2012 Dec 15;189(12):5638-48. doi: 10.4049/jimmunol.1200936. Epub 2012 Nov 2. J Immunol. 2012. PMID: 23125413 Free PMC article. - Deeper insight into the role of IL-17 in the relationship beween hypertension and intestinal physiology.
Yang ZJ, Wang TT, Wang BY, Gao H, He CW, Shang HW, Lu X, Wang Y, Xu JD. Yang ZJ, et al. J Inflamm (Lond). 2022 Oct 4;19(1):14. doi: 10.1186/s12950-022-00311-0. J Inflamm (Lond). 2022. PMID: 36195874 Free PMC article. Review. - Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis.
Abarbanel DN, Seki SM, Davies Y, Marlen N, Benavides JA, Cox K, Nadeau KC, Cox KL. Abarbanel DN, et al. J Clin Immunol. 2013 Feb;33(2):397-406. doi: 10.1007/s10875-012-9801-1. Epub 2012 Oct 9. J Clin Immunol. 2013. PMID: 23054338 Free PMC article. - Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis.
Sivaprakasam S, Prasad PD, Singh N. Sivaprakasam S, et al. Pharmacol Ther. 2016 Aug;164:144-51. doi: 10.1016/j.pharmthera.2016.04.007. Epub 2016 Apr 23. Pharmacol Ther. 2016. PMID: 27113407 Free PMC article. Review. - Manipulating resident microbiota to enhance regulatory immune function to treat inflammatory bowel diseases.
Mishima Y, Sartor RB. Mishima Y, et al. J Gastroenterol. 2020 Jan;55(1):4-14. doi: 10.1007/s00535-019-01618-1. Epub 2019 Sep 3. J Gastroenterol. 2020. PMID: 31482438 Free PMC article. Review.
References
- Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. - PubMed
- Fuss IJ, Boirivant M, Lacy B, et al. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J.Immunol. 2002;168:900–908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous