Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke - PubMed (original) (raw)
Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke
Adam P Mecca et al. Exp Physiol. 2011 Oct.
Abstract
Activation of angiotensin-converting enzyme 2 (ACE2), production of angiotensin-(1-7) [Ang-(1-7)] and stimulation of the Ang-(1-7) receptor Mas exert beneficial actions in various peripheral cardiovascular diseases, largely through opposition of the deleterious effects of angiotensin II via its type 1 receptor. Here we considered the possibility that Ang-(1-7) may exert beneficial effects against CNS damage and neurological deficits produced by cerebral ischaemic stroke. We determined the effects of central administration of Ang-(1-7) or pharmacological activation of ACE2 on the cerebral damage and behavioural deficits elicited by endothelin-1 (ET-1)-induced middle cerebral artery occlusion (MCAO), a model of cerebral ischaemia. The results of the present study demonstrated that intracerebroventricular infusion of either Ang-(1-7) or an ACE2 activator, diminazine aceturate (DIZE), prior to and following ET-1-induced MCAO significantly attenuated the cerebral infarct size and neurological deficits measured 72 h after the insult. These beneficial actions of Ang-(1-7) and DIZE were reversed by co-intracerebroventricular administration of the Mas receptor inhibitor, A-779. Neither the Ang-(1-7) nor the DIZE treatments altered the reduction in cerebral blood flow elicited by ET-1. Lastly, intracerebroventricular administration of Ang-(1-7) significantly reduced the increase in inducible nitric oxide synthase mRNA expression within the cerebral infarct that occurs following ET-1-induced MCAO. This is the first demonstration of cerebroprotective properties of the ACE2-Ang-(1-7)-Mas axis during ischaemic stroke, and suggests that the mechanism of the Ang-(1-7) protective action includes blunting of inducible nitric oxide synthase expression.
Figures
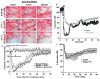
Figure 1. Effects of angiotensin-(1–7) [Ang-(1–7)] on endothelin-1 (ET-1)-induced middle cerebral artery (MCA) constriction and cerebral blood flow
A_–_I are photomicrographs showing the MCA branches (arrows) during 80 _μ_M ET-1-induced vasoconstriction. Primary and secondary branches of the MCA were visualized with a surgical microscope after temporal craniotomy to create a cranial window. Images were captured at a rate of 1 min−1, starting immediately prior to ET-1 injection (0 min), throughout the ET-1 injection (3 min) and for at least 60 min after initiation of the ET-1 injection. Representative images are shown for rats that underwent 7 days of intracerebroventricular (I.C.V.) artificial cerebrospinal fluid (aCSF) pretreatment (0.5 _μ_l h−1) prior to a sham middle cerebral artery occlusion [MCAO; 3 _μ_l of 0.9% saline (Sal) injection, _A_–_C_], 7 days of I.C.V. aCSF pretreatment (0.5 _μ_l h−1) prior to an ET-1-induced MCAO (3 _μ_l of 80 _μ_M ET-1 injection, D_–_F), or 7 days of I.C.V. Ang-(1–7) (1.1 nM; 0.5 μ_l h−1) pretreatment prior to an ET-1-induced MCAO (G_–_I). J shows vasoconstriction of primary or secondary MCA branches quantified as the percentage of baseline vessel diameter. Data are means ± SEM from four rats per treatment condition. Baseline vessel diameter was determined prior to ET-1 or 0.9% saline injection (time 0 min). *P < 0.05 for aCSF versus sham MCAO and †_P < 0.05 for Ang-(1–7) versus sham MCAO [two-way repeated-measures ANOVA (P < 0.001) followed by Bonferroni post hoc test]. K shows cerebral blood flow (CBF) in the ischaemic core and penumbra during ET-1-induced (MCAO). Laser Doppler flowmetry was used to investigate the CBF reduction that results in cortical areas both adjacent (ischaemic core) and distal (ischaemic penumbra) to the site of ET-1 (80 _μ_M) injection. Representative traces from one rat are shown for each probe position. The data are presented as a percentage of the baseline signal. L shows the effects of I.C.V. infusion of Ang-(1–7) on CBF in the vascular territory of the MCA distal to the site of ET-1 injection. Data are means ± SEM of the percentage change from baseline CBF. Endothelin-1 injection took place over a period of 3 min starting at 0 min on this graph. No significant differences exist between Ang-(1–7) (n = 6) and aCSF (n = 6) treatment groups at any time point (two-way repeated-measures ANOVA).
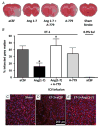
Figure 2. Intracerebral pretreatment with Ang-(1–7) reduces CNS infarct size after ET-1-induced MCAO
Rats were pretreated via the I.C.V. route with Ang-(1–7) (1.1 nM; 0.5 _μ_l h−1; n = 9), aCSF (n = 23), Ang-(1–7) + A-779 (1.14 nM; 0.5 μ_l h−1; n = 9) or A-779 (n = 7) alone for 7 days prior to MCAO induced by intracranial injection of ET-1 as described in the Methods. In addition, two rats received a sham MCAO with 0.9% saline injection in place of ET-1. Brains were removed for TTC staining 72 h after the ET-1-induced MCAO. A, representative brain sections showing infarcted (white) and non-infarcted grey matter (red) in the treatment conditions indicated. B, bar graphs are means + SEM showing the percentage infarcted grey matter in each treatment group. *P < 0.05 versus aCSF; †_P < 0.05 versus Ang-(1–7). C, D and E are representative fluorescence micrographs showing NeuN immunoreactivity co-localized with DAPI nuclear stain (pink coloured cells; red NeuN + blue DAPI) in the cerebral cortex of rats that underwent sham stroke surgery, ET-1-induced MCAO + I.C.V. infusion of aCSF (0.5 _μ_l h−1; 7 days) or ET-1-induced MCAO + I.C.V. infusion of Ang-(1–7) (1.1 nM; 0.5 _μ_l h−1; 7 days).
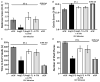
Figure 3. Intracerebral pretreatment with Ang-(1–7) reduces behavioural deficits 72 h after ET-1-induced MCAO
Rats were pretreated via the I.C.V. route with Ang-(1–7) (1.1 nM; 0.5 _μ_l h−1; n = 9), aCSF (n = 23), Ang-(1–7) + A-779 (1.14 nM; 0.5 μ_l h−1; n = 9) or A-779 (n = 7) alone for 7 days prior to ET-1-induced MCAO, as described in the Methods. The bar graphs shown here are the means ± SEM of data obtained from the Bederson neurological examination (A) and the Garcia neurological examination (B), as well as the sunflower seed-eating test for the time to eat five seeds (C) and the number of shell pieces (D). Also shown in each panel are data from two rats that underwent I.C.V. infusion of aCSF as above and a sham stroke (0.9% saline injection instead of ET-1). *P < 0.01 versus aCSF/ET-1 group; †_P < 0.01 versus Ang-(1–7) group.
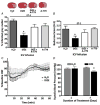
Figure 4. Intracerebral pretreatment with diminazine aceturate (DIZE) reduces CNS infarct size and behavioural deficits after ET-1-induced MCAO
Rats were pretreated via the I.C.V. route with water (DIZE solvent; n = 15), DIZE (5 _μ_g (0.5 _μ_l)−1 h−1); n = 18), DIZE + A-779 (1.14 nM; 0.5 _μ_l h−1; n = 7) or A-779 (n = 10) alone for 7 days prior to MCAO induced by intracranial injection of ET-1 (80 _μ_M). A, brains were removed for TTC staining 72 h after stroke. Representative brain sections (top) show infarcted (white) and non-infarcted grey matter (red) in the above-described treatment conditions. Bar graphs (bottom) are means + SEM showing the percentage infarcted grey matter in each treatment group. *P < 0.001 versus water. B, bar graphs are means + SEM showing the Bederson examination scores in each treatment condition, 72 h following the ET-1-induced MCAO. *P < 0.05 versus water. C, effects of I.C.V. infusion of DIZE (5 _μ_g (0.5 _μ_l)−1 h−1) on CBF in the vascular territory of the MCA distal to the site of ET-1 injection. Data are means ± SEM of the percentage change from baseline CBF. Endothelin-1 injection took place over a period of 3 min starting at 0 min. No significant differences exist between DIZE (n = 6) and water (n = 6) treatment groups at any time point (two-way repeated-measures ANOVA). D, effects of I.C.V. infusion of DIZE (5 _μ_g (0.5 _μ_l)−1 h−1) on systolic blood pressure. Bar graphs are means + SEM (n = 6 rats per group). *P < 0.01 versus water.
Figure 5. Intracerebral gene expression elicited by ET-1-induced MCAO: effects of Ang-(1–7)
A–E, rats were pretreated via I.C.V. infusion with either aCSF (0.5 μ_l h−1) or Ang-(1–7) (1.1 nM; 0.5 μ_l h−1) for 7 days prior to ET-1-induced MCAO or sham stroke (injection of 0.9% saline instead of ET-1). Twenty-four hours later, rats were killed, after which brains were removed and processed for qRT-PCR as detailed in the Methods. The bar graphs are means + SEM of Mas, AT1R, ACE2 iNOS, and IL-6 mRNA levels from the whole ipsilateral (right) hemisphere of each treatment group. *P < 0.05 versus corresponding sham treatment (n = 5–6 rats per group). F, rats were pretreated exactly as described for A_–_E. The bar graphs are means + SEM of iNOS mRNA levels from the ipsilateral (right) cerebral cortex stroke zone of each treatment group. *P < 0.05 versus corresponding sham treatment; †_P < 0.05 versus corresponding ET-1/aCSF group; (n = 5–8 rats per group). Note that these data are presented as (ΔΔ_Ct) values, thus a lower value represents an increase in mRNA level.
Comment in
- The angiotensin-converting enzyme 2-angiotensin-(1-7) axis: the other side of the renin-angiotensin system.
Shahid M. Shahid M. Exp Physiol. 2011 Oct;96(10):987-8. doi: 10.1113/expphysiol.2011.060335. Exp Physiol. 2011. PMID: 21914857 No abstract available.
Similar articles
- Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke.
Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C. Regenhardt RW, et al. Neuropharmacology. 2013 Aug;71:154-63. doi: 10.1016/j.neuropharm.2013.03.025. Epub 2013 Apr 11. Neuropharmacology. 2013. PMID: 23583926 Free PMC article. - Activation of the Neuroprotective Angiotensin-Converting Enzyme 2 in Rat Ischemic Stroke.
Bennion DM, Haltigan EA, Irwin AJ, Donnangelo LL, Regenhardt RW, Pioquinto DJ, Purich DL, Sumners C. Bennion DM, et al. Hypertension. 2015 Jul;66(1):141-8. doi: 10.1161/HYPERTENSIONAHA.115.05185. Epub 2015 May 4. Hypertension. 2015. PMID: 25941346 Free PMC article. - Neuroprotection by post-stroke administration of an oral formulation of angiotensin-(1-7) in ischaemic stroke.
Bennion DM, Jones CH, Donnangelo LL, Graham JT, Isenberg JD, Dang AN, Rodriguez V, Sinisterra RDM, Sousa FB, Santos RAS, Sumners C. Bennion DM, et al. Exp Physiol. 2018 Jun;103(6):916-923. doi: 10.1113/EP086957. Exp Physiol. 2018. PMID: 29663576 - Cerebroprotective action of angiotensin peptides in stroke.
Regenhardt RW, Bennion DM, Sumners C. Regenhardt RW, et al. Clin Sci (Lond). 2014 Feb;126(3):195-205. doi: 10.1042/CS20130324. Clin Sci (Lond). 2014. PMID: 24102099 Free PMC article. Review. - Angiotensin-(1-7): beyond the cardio-renal actions.
Passos-Silva DG, Verano-Braga T, Santos RA. Passos-Silva DG, et al. Clin Sci (Lond). 2013 Apr;124(7):443-56. doi: 10.1042/CS20120461. Clin Sci (Lond). 2013. PMID: 23249272 Review.
Cited by
- 2020 update on the renin-angiotensin-aldosterone system in pediatric kidney disease and its interactions with coronavirus.
Simões E Silva AC, Lanza K, Palmeira VA, Costa LB, Flynn JT. Simões E Silva AC, et al. Pediatr Nephrol. 2021 Jun;36(6):1407-1426. doi: 10.1007/s00467-020-04759-1. Epub 2020 Sep 29. Pediatr Nephrol. 2021. PMID: 32995920 Free PMC article. Review. - Impact of the Renin-Angiotensin System on the Pathogeny and Pharmacotherapeutics of Neurodegenerative Diseases.
Bild W, Vasincu A, Rusu RN, Ababei DC, Stana AB, Stanciu GD, Savu B, Bild V. Bild W, et al. Biomolecules. 2022 Oct 6;12(10):1429. doi: 10.3390/biom12101429. Biomolecules. 2022. PMID: 36291638 Free PMC article. Review. - Angiotensin converting enzyme 2: a new important player in the regulation of glycemia.
Chhabra KH, Chodavarapu H, Lazartigues E. Chhabra KH, et al. IUBMB Life. 2013 Sep;65(9):731-8. doi: 10.1002/iub.1190. Epub 2013 Jul 29. IUBMB Life. 2013. PMID: 23893738 Free PMC article. Review. - COVID-19-Related Stroke.
Hess DC, Eldahshan W, Rutkowski E. Hess DC, et al. Transl Stroke Res. 2020 Jun;11(3):322-325. doi: 10.1007/s12975-020-00818-9. Epub 2020 May 7. Transl Stroke Res. 2020. PMID: 32378030 Free PMC article. - Hi1a Improves Sensorimotor Deficit following Endothelin-1-Induced Stroke in Rats but Does Not Improve Functional Outcomes following Filament-Induced Stroke in Mice.
Knezic A, Budusan E, Saez NJ, Broughton BRS, Rash LD, King GF, Widdop RE, McCarthy CA. Knezic A, et al. ACS Pharmacol Transl Sci. 2024 Mar 14;7(4):1043-1054. doi: 10.1021/acsptsci.3c00328. eCollection 2024 Apr 12. ACS Pharmacol Transl Sci. 2024. PMID: 38638162
References
- Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am J Physiol Heart Circ Physiol. 2007;293:H1416–H1424. - PubMed
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
- Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H666–H672. - PubMed
- Castro-Chaves P, Cerqueira R, Pintalhao M, Leite-Moreira AF. New pathways of the renin-angiotensin system: the role of ACE2 in cardiovascular pathophysiology and therapy. Expert Opin Ther Targets. 2010;14:485–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL-083810/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 HL083810/HL/NHLBI NIH HHS/United States
- F30 NS060335/NS/NINDS NIH HHS/United States
- F30 NS060335-03/NS/NINDS NIH HHS/United States
- R01 HL110170/HL/NHLBI NIH HHS/United States
- F30 NS-060335/NS/NINDS NIH HHS/United States
- R01 DK090730/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous