Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88 - PubMed (original) (raw)
Comparative Study
. 2011 Jul 5;108(27):11169-74.
doi: 10.1073/pnas.1107941108. Epub 2011 Jun 20.
Samuele Calabro, Laura Santini, Barbara Galli, Alessia Genovese, Sara Valentini, Susanna Aprea, Annalisa Colaprico, Ugo D'Oro, Marzia M Giuliani, Michele Pallaoro, Mariagrazia Pizza, Derek T O'Hagan, Andreas Wack, Rino Rappuoli, Ennio De Gregorio
Affiliations
- PMID: 21690334
- PMCID: PMC3131326
- DOI: 10.1073/pnas.1107941108
Comparative Study
Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88
Anja Seubert et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Oil-in-water emulsions have been successfully used to increase the efficacy, immunogenicity, and cross-protection of human vaccines; however, their mechanism of action is still largely unknown. Nlrp3 inflammasome has been previously associated to the activity of alum, another adjuvant broadly used in human vaccines, and MyD88 adaptor protein is required for the adjuvanticity of most Toll-like receptor agonists. We compared the contribution of Nlrp3 and MyD88 to the adjuvanticity of alum, the oil-in-water emulsion MF59, and complete Freund's adjuvant in mice using a three-component vaccine against serogroup B Neisseria meningitidis (rMenB). Although the basal antibody responses to the nonadjuvanted rMenB vaccine were largely dependent on Nlrp3, the high-level antibody responses induced by alum, MF59, or complete Freund's adjuvant did not require Nlrp3. Surprisingly, we found that MF59 requires MyD88 to enhance bactericidal antibody responses to the rMenB vaccine. Because MF59 did not activate any of the Toll-like receptors in vitro, we propose that MF59 requires MyD88 for a Toll-like receptor-independent signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
IL-1β cleavage and release from BM-DC. BM-DC were primed overnight with serial dilutions of LPS (10 ng/mL, 1 ng/mL, 0.1 ng/mL, 0 ng/mL). MF59 or alum were added to the cells at the indicated concentrations and cells were incubated for further 24 h. Cleavage of pro–IL-1β was assessed by Western blotting (A) (M: protein weight marker) and cytokine release into the culture supernatant was measured by multiplex-bead ELISA (B). BM-DC from Nlrp3−/− or WT mice were primed with 10 ng/mL of LPS and incubated overnight with MF59 (1:100, vol:vol) or alum (400 μg/mL) (C).
Fig. 2.
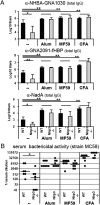
Antibody titers in Nlrp3−/− mice and WT controls after vaccination with plain or adjuvanted MenB antigens. (A) Total IgG antibody titers to all antigens contained in the vaccine: NHBA-GNA1030, GNA2091-fHBP, and NadA. Values represent means of Log10 titers of eight mice per group plus SD. Unpaired, two-tailed Student's t test was performed comparing WT vs. Nlrp3−/− and adjuvant vs. PBS injection (in WT mice). *P < 0.05 are considered significant and **P < 0.001 highly significant. (B) SBA of mouse polyclonal antibodies in the serum of eight single mice (black symbols) and their median value (line). Statistical analysis of WT vs. KO mice was performed using unpaired, two-tailed Mann-Whitney test. *P < 0.05.
Fig. 3.
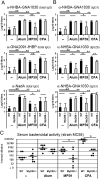
Antibody titers in MyD88−/− mice and WT controls after vaccination with plain or adjuvanted MenB antigens. (A) Total IgG antibody titers to all antigens contained in the vaccine: NHBA-GNA1030, GNA2091-fHBP, and NadA. (B) IgG subclasses IgG1, IgG2b, and IgG3 for one representative antigen (NHBA-GNA1030). Values represent means of Log10 titers of eight mice per group plus SD. Unpaired, two-tailed Student's t test. *P < 0.05; **P < 0.01. (C) SBA of mouse polyclonal antibodies in the serum of eight single mice (black symbols) and their median value (line). Unpaired, two-tailed Mann-Whitney test. *P < 0.05.
Fig. 4.
Innate immune responses after adjuvant stimulation in MyD88−/− mice or WT controls. (A) Cytokine content in intraperitoneal washfluid 4 h after intraperitoneal adjuvant injection. Black bars: WT mice; gray bars: MyD88−/− mice. Values represent mean of three mice plus SD. Unpaired, two-tailed Student's t test was performed comparing WT vs. MyD88−/− and adjuvant vs. PBS injection (in WT mice). *P < 0.05 **P < 0.001. (B) Cytokine content in serum at different timepoints postinjection of MF59. Black symbols: WT mice; gray symbols: MyD88−/− mice. Values represent mean of two to three mice ± SD. (C) Cell recruitment events in the peritoneal cavity 4 h after adjuvant injection. Values are given per 10E6 total peritoneal cells and represent means of three mice per group plus SD.
Similar articles
- Mechanism of action of licensed vaccine adjuvants.
Tritto E, Mosca F, De Gregorio E. Tritto E, et al. Vaccine. 2009 May 26;27(25-26):3331-4. doi: 10.1016/j.vaccine.2009.01.084. Epub 2009 Feb 5. Vaccine. 2009. PMID: 19200813 - Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59.
Ellebedy AH, Lupfer C, Ghoneim HE, DeBeauchamp J, Kanneganti TD, Webby RJ. Ellebedy AH, et al. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2927-32. doi: 10.1073/pnas.1012455108. Epub 2011 Jan 26. Proc Natl Acad Sci U S A. 2011. PMID: 21270336 Free PMC article. - The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination.
Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, Palmieri E, Pallaoro M, Rappuoli R, Di Virgilio F, De Gregorio E, Montecucco C, Seubert A. Vono M, et al. Proc Natl Acad Sci U S A. 2013 Dec 24;110(52):21095-100. doi: 10.1073/pnas.1319784110. Epub 2013 Dec 9. Proc Natl Acad Sci U S A. 2013. PMID: 24324152 Free PMC article. - MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis.
Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. Banzhoff A, et al. Influenza Other Respir Viruses. 2008 Nov;2(6):243-9. doi: 10.1111/j.1750-2659.2008.00059.x. Influenza Other Respir Viruses. 2008. PMID: 19453401 Free PMC article. Review. - Immunology of TLR-independent vaccine adjuvants.
De Gregorio E, D'Oro U, Wack A. De Gregorio E, et al. Curr Opin Immunol. 2009 Jun;21(3):339-45. doi: 10.1016/j.coi.2009.05.003. Epub 2009 Jun 1. Curr Opin Immunol. 2009. PMID: 19493664 Review.
Cited by
- Mechanisms of action of adjuvants.
Awate S, Babiuk LA, Mutwiri G. Awate S, et al. Front Immunol. 2013 May 16;4:114. doi: 10.3389/fimmu.2013.00114. eCollection 2013. Front Immunol. 2013. PMID: 23720661 Free PMC article. - Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes.
Powell BS, Andrianov AK, Fusco PC. Powell BS, et al. Clin Exp Vaccine Res. 2015 Jan;4(1):23-45. doi: 10.7774/cevr.2015.4.1.23. Epub 2015 Jan 30. Clin Exp Vaccine Res. 2015. PMID: 25648619 Free PMC article. Review. - Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus.
Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, Beutler B, Theofilopoulos AN. Baccala R, et al. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):2940-5. doi: 10.1073/pnas.1222798110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382217 Free PMC article. - Influenza Virus and SARS-CoV-2 Vaccines.
Sandor AM, Sturdivant MS, Ting JPY. Sandor AM, et al. J Immunol. 2021 Jun 1;206(11):2509-2520. doi: 10.4049/jimmunol.2001287. Epub 2021 May 21. J Immunol. 2021. PMID: 34021048 Free PMC article. Review. - Size matters in non-canonical inflammasome activation and cell-mediated immunity.
Weiss AM, Esser-Kahn AP. Weiss AM, et al. Cell Rep Med. 2023 Jan 17;4(1):100904. doi: 10.1016/j.xcrm.2022.100904. Cell Rep Med. 2023. PMID: 36652913 Free PMC article.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. - PubMed
- Morefield GL, et al. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588–1595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases